Assessing the Stability of Alanine-Based Helices by Conformer-Selective IR Spectroscopy
Investigating the H-bond network of polyalanine-based peptides
News from Jul 05, 2016
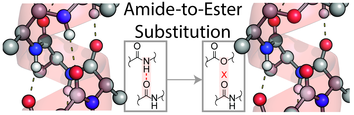
In our newest publication we investigate polyalanine-based peptides that carry a lysine at the C-terminus, which are known to form α-helices in the gas phase. Three factors contribute to the stability of these helices: ɪ) the interaction between the helix macro dipole and the charge, ɪɪ) the capping of dangling C=O groups by lysine and ɪɪɪ) the cooperative hydrogen bond network. In previous studies, the influence of the interaction between the helix dipole and the charge as well as the impact of the capping was studied intensively. Here, we complement these findings by systematically assessing the third parameter, the H-bond network. In order to selectively remove one H-bond along the backbone, we use amide-to-ester substitutions. The resulting depsi peptides were analyzed by ion-mobility and m/z-selective infrared spectroscopy as well as theoretical calculations. Our results indicate that peptides which contain only one ester bond still maintain the helical conformation. We conclude that the interaction between the charge and the helix macro-dipole is most crucial for the formation of the α-helical conformation and a single backbone H-bond has only little influence on the overall stability.
go to article DOI: 10.1039/C6CP03827A




