New Publication in JACS
The Influence of the Electron Density in Acyl Protecting Groups on the Selectivity of Galactose Formation
News from Nov 09, 2022
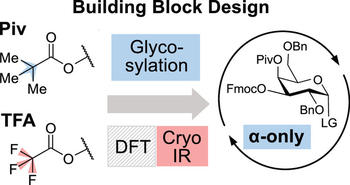
The stereoselective formation of 1,2-cis-glycosidic bonds is a major bottleneck in the synthesis of carbohydrates. We here investigate how the electron density in acyl protecting groups influences the stereoselectivity by fine-tuning the efficiency of remote participation. Electron-rich C4-pivaloylated galactose building blocks show an unprecedented α-selectivity. The trifluoroacetylated counterpart with electron-withdrawing groups, on the other hand, exhibits a lower selectivity. Cryogenic infrared spectroscopy in helium nanodroplets and density functional theory calculations revealed the existence of dioxolenium-type intermediates for this reaction, which suggests that remote participation of the pivaloyl protecting group is the origin of the high α-selectivity of the pivaloylated building blocks. According to these findings, an α-selective galactose building block for glycosynthesis is developed based on rational considerations and is subsequently employed in automated glycan assembly exhibiting complete stereoselectivity. Based on the obtained selectivities in the glycosylation reactions and the results from infrared spectroscopy and density functional theory, we suggest a mechanism by which these reactions could proceed.
go to article: https://pubs.acs.org/doi/10.1021/jacs.2c05859