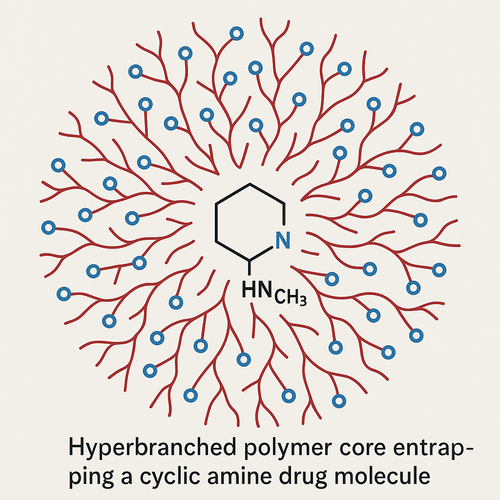
Hyperbranched polyester polyol derivative as drug solubilizer
A sulfated hyperbranched polyester polyol selectively encapsulates hydrophobic small molecules bearing at least one amine, dramatically boosting aqueous solubility. The two-step, one-pot synthesis uses only glycidol, ε-caprolactone, and a sulfation reagent—no organic solvents—yielding a scalable solubilizing platform.
Innovation Explanation
This invention addresses a key pharma hurdle: many intracellular targets require hydrophobic, medium-MW drugs that suffer poor water solubility and bioavailability. By copolymerizing glycidol with ε-caprolactone at 40–140 °C and then sulfating the resulting hyperbranched polyester polyol, a dendritic carrier emerges that protonates cyclic or aliphatic amines and entraps them in its hydrophobic core. Aqueous formulations of this polymer freely solubilize diverse kinase inhibitors (e.g., sunitinib, trametinib) by up to 100-fold over water alone. Formulations can be purified via centrifugation and size-exclusion chromatography, then lyophilized with minimal impact on particle size or charge, and without organic solvents. In vitro assays confirm retained bioactivity, and the freeze-dried solids exhibit extended shelf life versus prior art micelles or surfactants.
Key Innovation Features and Advantages
- Selective solubilization: Efficient loading of drugs only if they carry an amine group, avoiding nonspecific binding.
- Organic-solvent-free synthesis: Simplifies scale-up and regulatory compliance.
- High loading capacity: Carrying potentials up to ~12 wt% demonstrated by elemental analysis and UV-Vis.
- Broad applicability: Suits oral, parenteral, nasal, pulmonary, ocular, and other routes.
- Stable lyophilization: Maintains size (12–14 nm) and zeta potential (–20 to –35 mV).
- Tunable structure: Glycidol/ε-caprolactone ratio and temperature tailor core hydrophobicity and surface charge.
Use Cases
- Rapid oral formulations of poorly soluble oncology drugs (e.g., dabrafenib, osimertinib).
- Parenteral delivery of kinase inhibitors requires high blood concentrations.
- Fixed-dose combinations of multiple hydrophobic agents for synergistic therapies.
- Lyophilized drug-polymer powders for stable transport and on-site reconstitution.
Commercial Opportunities
- Licensing platform to pharma for next-gen solubilizers. – CDMO production of API-polymer complexes under cGMP.
- CDMO production of API-polymer complexes under cGMP.
- Co-formulation services for NCEs struggling with solubility.
- Asset spin-out for targeted oncology drug delivery.
Current Status
Patent filed as WO 2021/152172 A1; experimental proof-of-concept complete with DLS, UV-Vis, EA, and cell-viability (IC₅₀) assays. Scale-up and in vivo PK/PD studies are underway.

Keywords
- hyperbranched polyester, dendritic solubilizer, hydrophobic drug, amine-bearing small molecule, organic-solvent-free, freeze-dryable, oral bioavailability