Structure and function of TGF-beta family receptor complexes
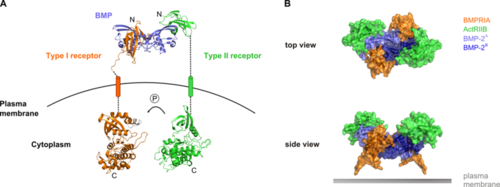
Fig. 1. A. Overall side view of the BMP receptor signaling complex B. Top view and side view of the heterohexameric extracellular complex comprising the dimeric BMP and each two type I and type II receptor molecules
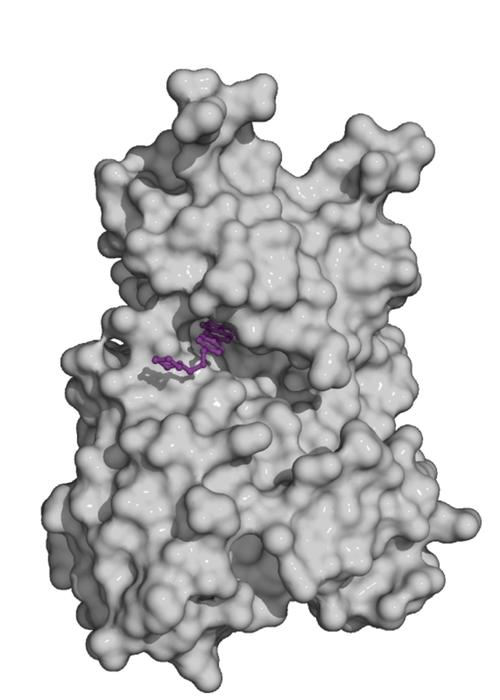
Fig. 2. Kinase domain of the TGF-beta family type II receptor ActRIIA (grey) with the small molecule inhibitor dorsomorphin (violet) bound to the ATP pocket
Image Credit: Daniel Horbelt
Cytokines of the TGFβ superfamily, including BMPs, signal through a complex of type I and type II transmembrane receptors at the plasma membrane (Figure 1A). There are four different type I and three type II receptors for BMPs. Both types of receptor contain a disulphide-bonded extracellular domain that binds the BMP ligand, a transmembrane region, a juxtamembrane region and a kinase domain. The type I receptor also contains a glycine and serine-rich region (GS-box) adjacent to its kinase domain. In addition, one splice-form of the BMP type II receptor (BMPRII) has a large C-terminal extension comprising 508 amino acids after the kinase domain. This ‘tail’ region is known to be functionally important and mediates interactions with a plethora of intracellular proteins. To date, its structure has not yet been solved.
BMPs and TGFβs are active as covalent dimers, and bind to heterotetrameric complexes of type I and type II receptors (Fig. 1B). However, they have distinct modes of binding. While TGFβ induces assembly of the complex by binding to its type II receptor, BMPs can bind to pre-formed receptor complexes or induce complex formation. We previously showed that pre-formed complexes and BMP-induced signalling complexes exhibit different patterns of mobility within the plasma membrane. Moreover, they activate different downstream signalling events - the Smad and p38 MAPK pathways respectively. The structural basis of these signalling differences is unknown and we still lack a detailed understanding of how the downstream signalling molecules associate with the BMP receptor complexes.
Within the BMP receptor complex, the type I receptor is activated through phosphorylation of serine and threonine residues upstream of the kinase domain by the type II receptor (including the GS-box). In the canonical Smad pathway, receptor-activated Smads interact with the receptor complex and are phosphorylated at their extreme C-terminus by the active type I receptor kinase. Phosphorylated Smads accumulate in the nucleus to regulate transcription. In parallel to Smad pathways, other pathways can be initiated at the receptor complex that do not involve Smad proteins. Some superfamily ligands have been shown to induce phosphorylation of several MAP kinases as well as Akt/PKB, and to regulate the activity of signaling players such as Rho and LIMK
Our lab is addressing a series of open questions in the context of the activation of Smad and non-Smad pathways.
-
We are studying the formation of active ligand-receptor complexes which can form either upon binding of ligands to pre-formed complexes, or upon recruitment of free receptors to ligand-bound receptors. We are investigating these processes by looking at both the extracellular and cytosolic domains using a combination of live cell microscopy and biochemical assays.
-
The initiation of signalling pathways depends on proteins that associate with the receptor complex in its active or inactive conformation. These can be either components of downstream pathways, scaffold proteins or signalling repressors. We have identified a number of such proteins and characterised their function in the TGFβ superfamily-induced pathways, and we continue to explore novel players. We also aim to understand the molecular and structural basis of their interactions with the receptors.
-
Small molecule inhibitors for TGF-beta family receptors are widely used to block specific signalling pathways. They have proven their potential in signal transduction research and are being evaluated for therapeutical application. In either context, profound knowledge of their specificities and efficacies to target individual receptors of the family is critical. Together with our collaborators we are characterizing novel inhibitors for TGF-beta family signalling in comprehensive biochemical and cell-based studies.