Research topics
Our aim is to implement the following criteria into the general understanding of BMP signal transduction, not only in stem and precursor cells, but also fully differentiated cells: biomechanical stimulation, extracellular modulators, subcellular compartmentalization of signaling pathways and crosstalk to the Hippo and insulin signaling cascades.
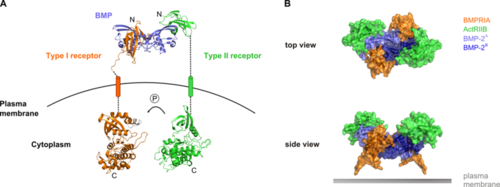
Fig. 1. A. Overall side view of the BMP receptor signaling complex B. Top view and side view of the heterohexameric extracellular complex comprising the dimeric BMP and each two type I and type II receptor molecules
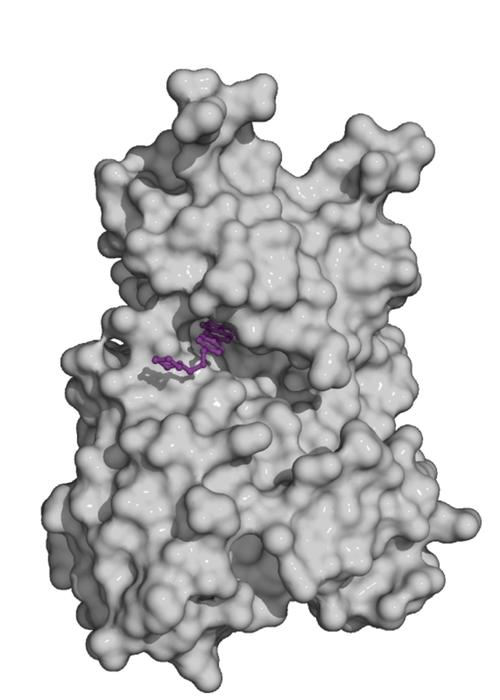
Fig. 2. Kinase domain of the TGF-beta family type II receptor ActRIIA (grey) with the small molecule inhibitor dorsomorphin (violet) bound to the ATP pocket
Image Credit: Daniel Horbelt
Cytokines of the TGFβ superfamily, including BMPs, signal through a complex of type I and type II transmembrane receptors at the plasma membrane (Figure 1A). There are four different type I and three type II receptors for BMPs. Both types of receptor contain a disulphide-bonded extracellular domain that binds the BMP ligand, a transmembrane region, a juxtamembrane region and a kinase domain. The type I receptor also contains a glycine and serine-rich region (GS-box) adjacent to its kinase domain. In addition, one splice-form of the BMP type II receptor (BMPRII) has a large C-terminal extension comprising 508 amino acids after the kinase domain. This ‘tail’ region is known to be functionally important and mediates interactions with a plethora of intracellular proteins. To date, its structure has not yet been solved.
BMPs and TGFβs are active as covalent dimers, and bind to heterotetrameric complexes of type I and type II receptors (Fig. 1B). However, they have distinct modes of binding. While TGFβ induces assembly of the complex by binding to its type II receptor, BMPs can bind to pre-formed receptor complexes or induce complex formation. We previously showed that pre-formed complexes and BMP-induced signalling complexes exhibit different patterns of mobility within the plasma membrane. Moreover, they activate different downstream signalling events - the Smad and p38 MAPK pathways respectively. The structural basis of these signalling differences is unknown and we still lack a detailed understanding of how the downstream signalling molecules associate with the BMP receptor complexes.
Within the BMP receptor complex, the type I receptor is activated through phosphorylation of serine and threonine residues upstream of the kinase domain by the type II receptor (including the GS-box). In the canonical Smad pathway, receptor-activated Smads interact with the receptor complex and are phosphorylated at their extreme C-terminus by the active type I receptor kinase. Phosphorylated Smads accumulate in the nucleus to regulate transcription. In parallel to Smad pathways, other pathways can be initiated at the receptor complex that do not involve Smad proteins. Some superfamily ligands have been shown to induce phosphorylation of several MAP kinases as well as Akt/PKB, and to regulate the activity of signaling players such as Rho and LIMK
Our lab is addressing a series of open questions in the context of the activation of Smad and non-Smad pathways.
-
We are studying the formation of active ligand-receptor complexes which can form either upon binding of ligands to pre-formed complexes, or upon recruitment of free receptors to ligand-bound receptors. We are investigating these processes by looking at both the extracellular and cytosolic domains using a combination of live cell microscopy and biochemical assays.
-
The initiation of signalling pathways depends on proteins that associate with the receptor complex in its active or inactive conformation. These can be either components of downstream pathways, scaffold proteins or signalling repressors. We have identified a number of such proteins and characterised their function in the TGFβ superfamily-induced pathways, and we continue to explore novel players. We also aim to understand the molecular and structural basis of their interactions with the receptors.
-
Small molecule inhibitors for TGF-beta family receptors are widely used to block specific signalling pathways. They have proven their potential in signal transduction research and are being evaluated for therapeutical application. In either context, profound knowledge of their specificities and efficacies to target individual receptors of the family is critical. Together with our collaborators we are characterizing novel inhibitors for TGF-beta family signalling in comprehensive biochemical and cell-based studies.
Diversity in BMP signal transduction is achieved by activation of Smad and non‑Smad pathways including their mode of action in different subcellular compartments.
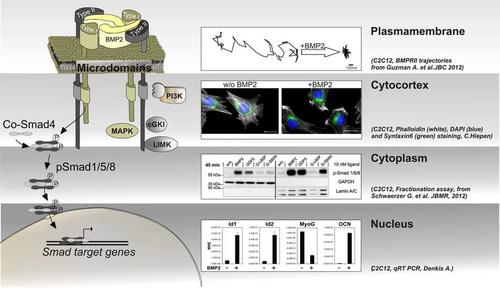
The Smad signalling cascade originates from pre-formed BMPRI and BMPRII receptor complexes and is fine tuned by and crosstalks to other pathways in the cytoplasm to finally act in the nucleus by activation of Smad dependent target genes. Non-Smad signalling, such as activation of MAPK, requires the formation of BMP-induced receptor complexes and may also act in different subcellular compartments, dependent on the cell context.
With respect to subcellular compartmentalization of BMP signalling, we therefore focus on:
- the plasma membrane, in particular the dynamics of BMP receptors within distinct membrane microdomains and the role of membrane scaffolding proteins in segregating and facilitating specific pathways. Here, we establish single particle tracking microscopy (SPT) of the receptors to follow the differential lateral mobility of BMPRI and BMPRII under defined stimulation conditions. Our current aim is to understand mechanisms of confinement, the establishment of diffusion barriers but also active transport mechanisms of BMP receptors to and from the plasma membrane and how this integrates into BMP signalling intensity, specificity and duration.
- the cytocortex which represents the most proximal cytoplasmic region below the plasma membrane of polarized cells. Here BMPs induce heavy and quick cytoskeletal rearrangements but also recruitment/tethering of scaffolding proteins and the production/enrichment of distinct lipids. Application of Mass Spectrometry revealed novel BMP receptor interacting proteins that produce lipids or show a lipid dependency but also act as cytoskeletal regulators. To investigate novel BMP dependent molecular mechanisms in respect to planar cell polarity, cell migration and endo-/exo-cytosis we use lightoptical techniques such as confocal-, super-resolution-, and TIRF-microscopy.
- the cytoplasm as well as cytoplasmic compartments such as endocytic or exocytic vesicles. Here we aim to understand where and when BMP pathway proteins are transported and fine tuned and how they integrate and crosstalk to other pathways such as Wnt, Notch or Hippo cascades. As shown by others, this crosstalk appears also on the level of Smad linker phosphorylation leading to degradation of Smads. We use proteomics and protein-protein biochemistry to identify novel regulatory frameworks as well as life cell imaging and biosensors to visualize translocation events in real time.
- the nucleus including the nuclear envelope. Entrance of Smads into the nucleus including assembly of transcriptional complexes pre or post entrance may fine tune the transcriptional regulation of Smad dependent target genes. In that respect we are interested in kinetics of Smad nuclear translocation but also into identifying novel Smad transcriptional complexes involved in osteogenic, myogenic and endothelial differentiation.
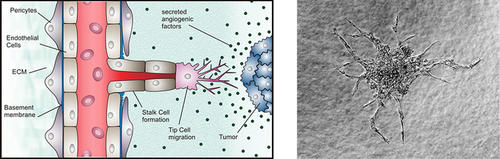
Currently we are investigating the contribution of distinct BMP receptors in the regulation of vascular integrity and in particular in the endothelium. As we have shown BMPR2/Alk2 receptor complexes modulate endothelial adherence junctions by triggering VE-cadherin internalization via src-kinase (Benn et al., JCS, 2016). Furthermore BMP type I receptors Alk3 and Alk2 both play crucial and distinct roles in sprouting angiogenesis. Tip cells express Alk3 and induce the tip cell markers DLL4 and KDR via the BMP2-p38 pathway, while Alk2 is expressed in stalk cells to induce stalk-cell genes via the BMP6-Smad1/5 pathway (Benn et al., FASEB J, 2017).
This unique and balanced expression profile of different BMP receptors is crucial in maintaining vessel integrity and to cope with mechanical and inflammatory stimuli.
Mutations within the gene of BMPR2 have been described in the rare disease pulmonary arterial hypertension (PAH). PAH is characterized by narrowing of blood vessels within the lung, which results in increased blood preasure from the heart. As a consequence the vessels become stiffer and thicker. As BMP signaling is sensed by alterations in cellular mechanical forces, a vicious cycle accelerates the development of the disease locally. We are currently investigating the impact of BMP receptor mutations in endothelial cells in the context of sensing mechanical cues derived from the extracellular matrix. For this we developed biophysical and bioreactor systems to develop novel drug screening platforms.
Fibrodysplasia ossificans progressive (FOP) is a severely disabling genetic disorder in which bone forms at extraskeletal soft tissue sites. It is a rare autosomal dominant disorder occurring at a population frequency of about one in 2 million. The two main characteristic clinical features of classic FOP are congenital malformations of the great toes and progressive, episodic heterotopic ossification (HO), called flare-up, at distant sites. The mutation R206H in Alk2 is most prevalent found in FOP patients and causes a hypersensitive signal transduction. Bone precursor cells leading to these HO are amongst others derived from the endothelium through a process called Endothelial-to-Mesenchymal Transition (EndMT). As we found that Alk2 plays a crucial role in endothelial barrier function our current research focus is to investigate this in the context of FOP and other rare diseases.
Coordinated tissue development and regeneration through balanced signaling cues is crucial for healthy tissue homeostasis.
BMPs are a major class of secreted metabologens that constitute pivotal morphogenetic signals and orchestrate general tissue homeostasis.
BMP signaling is fine-tuned at each stage of signal transduction, predominantly the BMP receptors. The extracellular BMP antagonist Noggin directly couples immediate and long-term environmental changes with the local and systemic operating mode of the cytokine. Thereby, BMP and Noggin form a mutually non-functional unit that abrogates extracellular BMP receptor binding, hence intracellular signal transduction.
Current knowledge suggests that deregulated local intra-tissue expression of BMP and Noggin impairs proliferative, apoptotic and differentiation processes with pathophysiological characteristics in bone, muscle and adipose tissue development. Interestingly, elevated circulating inter-tissue BMP and Noggin levels in adult mice and humans suggest that both factors play a putative role in energy balance and body weight regulation with consecutive implications on muscle and bone tissue homeostasis.
Therefore, it is suggested that besides BMP signaling, Noggin is a crucial factor in healthy bone, muscle and adipose tissue homeostasis. To understand the causal interrelation of how disintegrated local BMP signal adaptation affects systemic adjustments in the body we characterize diseases such as obesity and muscular atrophy through rare hereditary and frequent acquired pathophysiological mouse and human samples.
We use experimental and mathematical models which integrate cellular, tissue and systemic approaches to address whether imbalanced intra- and inter-tissue (ant)-agonist expression and functionality is the cause or the consequence of these conditions.
We aim to contribute to the molecular understanding of the pathomechanisms of obesity and muscular atrophy to provide potential new therapeutic approaches for the early detection of disease onset and progression and the causative treatment of consecutive diseases such as type 2 diabetes and osteoporosis.
A: Bone cells moving around on a culture surface by rearranging their cytoskeleton. B: Fluid flow leads to calcium influx into the cytoplasm. C: Mesenchymal cells migrate on soft hydrogels in a wound healing assay. (click image to play)
Bone Morphogenetic Proteins (BMPs) are important regulators in a multitude of cellular processes and their signaling pathway is tightly controlled by a regulatory network including not only biochemical factors but also mechanical cues. The abundance of mechanical forces in the body contributes to shaping and homeostasis of many tissues and cell types, such as endothelial cells, osteoblasts or muscle cells. Mechanical signals that control cell fate decisions may comprise active forces, such as compressive or shear forces, but may also be encoded by substrate characteristics like stiffness, geometry or ligand spacing.
The Knaus Lab investigates the interplay between BMP signaling and mechanotransduction mainly in three contexts: bone development and metabolism, physiological and pathological blood flow in the vascular system, and instruction of mesenchymal progenitors by mechanical signals encoded in the extracellular matrix. To address our research questions we apply several bioreactor- and biomaterial-based cell culture systems that allow the modulation of the mechanical microenvironment. We in particular aim to unravel the molecular principles integrating mechanical signals into BMP signaling cascades and focus on their physiological outcome in different cell systems. Specifically we are interested in crosstalks of BMP signaling to the cytoskeleton, adhesion sites, ion channels, and the cilium, as well as defining the role of the BMP receptors in mechanoresponsiveness of the cell. Further, we use hydrogel-based culture system to analyze how substrate properties such as stiffness, ligand presentation and integrin-ECM interactions shape the cellular response on a molecular and functional level.
A more detailed understanding of this crosstalk in respect to molecular interactions will be indispensable in the future, in particular to understand BMP-related diseases as well as with regard to an efficient clinical application of BMP ligands.
Methods:
- 2D and 3D culture of cells on substrates with varying stiffness, architecture, and biochemical functionalization
- High resolution microscopy of fixed and living cells
- Bioreactor systems to apply compressive and shear forces of different magnitudes
- Proteinbiochemical and expression analyses of cellular responses to different mechanical inputs
Collaboration partners:
- Dr. Ansgar Petersen, Zelluläre Biomechanik, Julius Wolff Institut für Biomechanik und Muskuloskeletale Regeneration, Charite, Berlin
- Associate Professor of Pediatrics Prof. Dr. Hans-Georg Simon, Stanley Manne Children's Research Institute, Northwestern University, Chicago
- Prof. Dr. Carsten Werner, Institute of Biofunctional Polymer Materials, Leibniz-Institut für Polymerforschung e.V. Dresden
Funding:
- Forschergruppe 2165 – Regeneration in Aged
- Berlin Brandenburg School for Regenerative Therapies
Scheme of tumor-induced angiogenesis (adapted from Carmeliet & Jain, Nature, 2011) and sprouting angiogenesis from a HUVEC spheroid (work by A. Benn, Knaus Lab)
Sprouting angiogenesis describes the formation of new blood vessels from pre-existing vasculature, a mechanism essential during embryonic development, but also in tumor-induced angiogenesis.
In the past, in vivo targeting of BMP pathway proteins gave rise to multiple vascular phenotypes uncovering BMP signaling to be essential for angiogenic processes by acting on cells that constitute blood vessels, such as endothelial cells and smooth muscle cells. Interestingly, recent research suggests BMPs to regulate endothelial cell function and induce sprouting angiogenesis independent of VEGF signals. However, a detailed molecular understanding of BMP signal transduction in the vascular system, termed ‘vascular BMP signaling’, is still missing and the individual contribution of the BMP-induced signaling pathways in this process is barely understood.
To address these questions we perform in vitro angiogenesis assays, as well as ex vivo organ culture models to characterize vascular BMP signaling. In particular, we are interested in elucidating the role of BMPs on sprouting angiogenesis and identify novel key effectors. We aim to gain a more accurate molecular understanding of vascular BMP signal transduction, the role of individual BMP receptors and related key signal transduction proteins. This detailed molecular knowledge is essential to the development of novel anti-angiogenic therapies but also to gain a better view on how BMPs orchestrate angiogenesis.
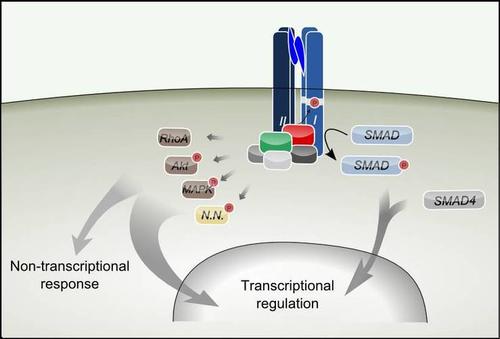
Fig. 1.TGF-beta superfamily ligands activate Smad and non-Smad pathways such as RhoA, Akt, and MAP Kinase (MAPK). There may be pathways which are yet to be discovered (N.N.).
Image Credit: Dr. Daniel Horbelt
Transforming growth factor-β (TGF-β) is a modulator in developmental processes and a regulator in homeostasis. Dysregulation of TGF-β signaling is associated with human diseases such as cancer and cardiovascular disease. Hereditary defects predispose to metabolic, musculoskeletal, and cardiovascular malfunctions.
Even though the principal functions and pathways of TGF-β signaling have been elucidated in the past decades, increasing levels of complexity regarding its role in vivo and its signaling mechanisms have emerged, emphasizing the highly context specific nature of this pathway.
TGF-β superfamily signaling is initiated by binding of an extracellular ligand to cognate receptors, which promotes the formation of active receptor signaling complexes. The signaling pathways that originate from these complexes, the canonical Smad pathway and several non-Smad pathways, trigger transcriptional regulation but also direct cellular responses (Fig. 1).
We are investigating the "signalotypes" of pathway components that exhibit an abnormal functionality as a consequence of disease-related inherited or somatic mutations. Mutations in TβRII (TGFBR2 gene) were found associated with a group of diseases that feature significant phenotypic similarity with Marfan syndrome (MFS). Our analysis of mutations in TβRII which are associated with Loeys-Dietz syndrome (LDS), type 2 Marfan syndrome (MFS2), or familial thoracic aortic aneurysms and dissections (FTAAD) revealed a correlation between clinical severity and signaling activity. Results from this study suggest that the balance of canonical TGF-ß-Smad signaling and non-canonical ERK signaling is a determinant for the development of thoracic aortic aneurysms and dissections (TAAD) on one hand and additional manifestations including skeletal and craniofacial phenotypes on the other hand.
The pathological consequences of mutant receptors, however, could not be fully explained to date on the basis of apparent defects in the receptor proteins themselves, such as altered expression, localisation, or enzymatic activity.
Thus, our most recent approaches to understand the consequences of pathological mutations in TGF-β receptors target levels further downstream, by identifying the differential interactomes of wildtype and mutant receptors. A series of receptor-interacting proteins have been identified which associate with the receptors in a context-dependent manner. Some of these proteins were demonstrated to modulate TGF-β signalling by either enhancing or repressing the signalling activity originating at the receptors. We have already generated the interactomes of various wildtype receptors of the TGF-β superfamily using different experimental strategies and have sucessfully identified and characterized novel regulators of TGF-β superfamily signalling.
By identifying proteins that selectively associate with wildtype or mutant TGF-β superfamily receptors, respectively, we will be able to elucidate mechanisms of disease development that are obscure to date.
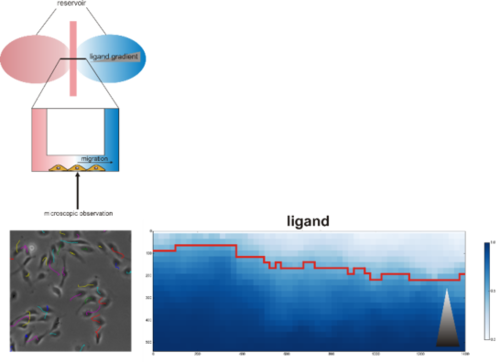
Top: Model of chemotaxis assay chamber (adapted from Ibidi). Left: Annotated trajectories for migrating cells. Right: Markov committor analysis of migratory behavior (gray triangle: growth factor gradient)
Directed cell migration is a complex process that involves cell polarization induced by chemical signals including growth factors, cell adhesion, formation of protrusions, retraction and contraction of a single cell or cell collective. The polarization depends on different mechanochemical signals including chemical compositions of the substrate, substrate stiffness, geometries and adhesive cues.
Migration is a highly coordinated process that involves synchronized rearrangements of cytoskeleton, dissolution of cell-cell contacts and polarization to orient the cell towards a targeted area.
The BMP signaling pathway is acknowledged to control migration of diverse cell types, including neural crest cells, mesenchymal progenitor cells and endothelial cells. The exact molecular mechanism is still unresolved. Cancer, hypoganglionosis, Hirschsprung's disease and intestinal neuronal dysplasia are pathologies with aberrant BMP signaling resulting in altered cell migration emphasizing the role of the pathway in migration.
Our lab investigates the mechanism by which BMPs induce (directional) migration/chemotaxis in various cell types and its functional implication in diseases. We use chemotaxis assays in which chemoattractant gradients are formed with simultaneous digital time lapse recording of cell movement to be able to track migration of cells in response to stimuli. Automated analysis of large data collections from these experiments are done in collaboration with the “Image analysis in biology and material science” at the Zuse Institute Berlin (ZIB) and enables us to track migration speed and orientation of single cells in response to chemical stimuli.