Structures solved in the AG Freund
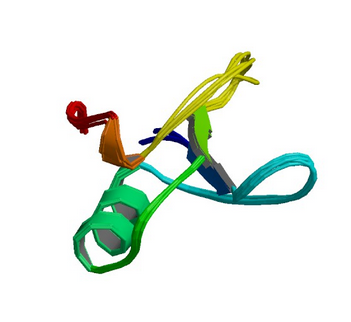
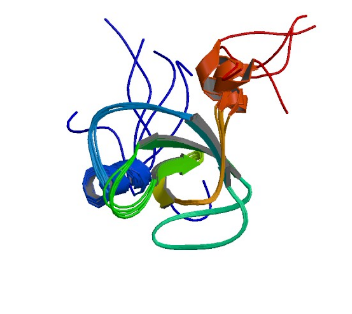
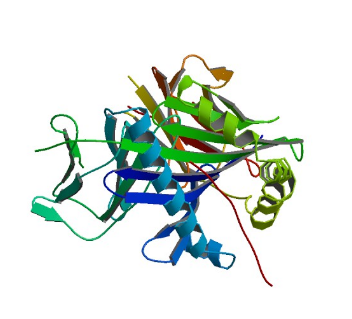
1GYF: GYF domain of human CD2BP2
Image from the RCSB PDB (www.rcsb.org) of PDB ID 1GYF (Freund, C., Doetsch, V., Nishizawa, K., Reinherz, E.L., Wagner, G.) (1999) The GYF domain is a novel structural fold that is involved in lymphoid signaling through proline-rich sequences. Nat.Struct.Biol. 6: 656-660
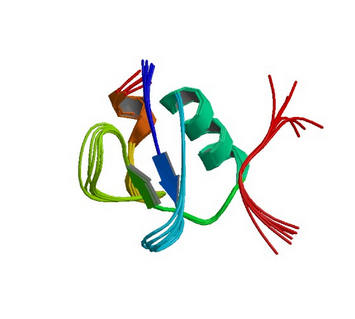
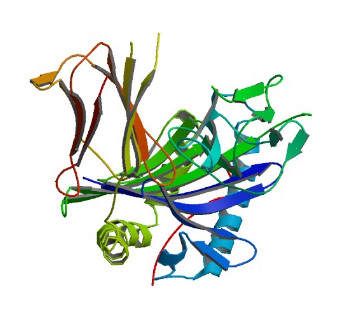
1L2Z: GYF domain of CD2BP2 in complex with proline-rich peptide
Image from the RCSB PDB (www.rcsb.org) of PDB ID 1L2Z (Freund, C., Kuhne, R., Yang, H., Park, S., Reinherz, E.L., Wagner, G.) (2002) Dynamic interaction of CD2 with the GYF and the SH3 domain of compartmentalized effector molecules Embo J. 21: 5985-5995
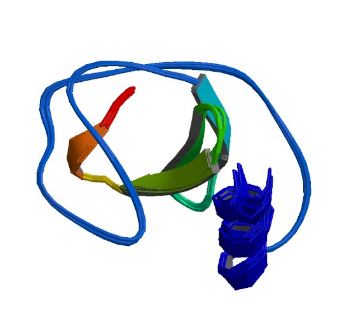
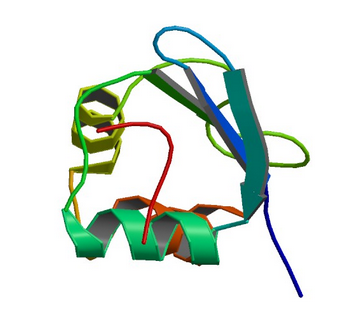
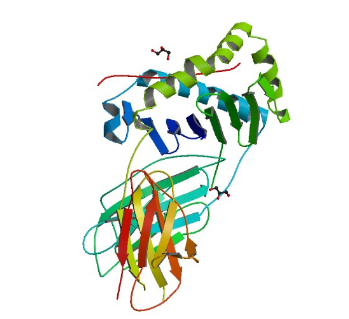
1RI9: hSH3C domain of the T cell protein ADAP
Image from the RCSB PDB (www.rcsb.org) of PDB ID 1RI9 (Heuer, K., Kofler, M., Langdon, G., Thiemke, K., Freund, C.) (2004) Structure of a Helically Extended SH3 Domain of the T Cell Adapter Protein ADAP. STRUCTURE 12: 603-610
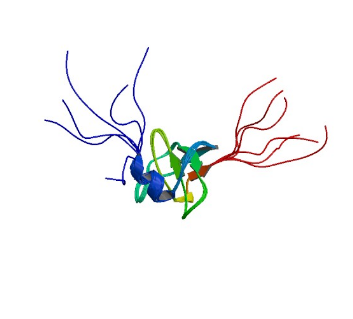
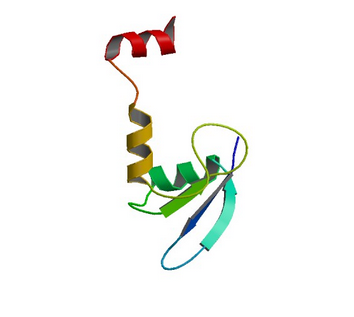
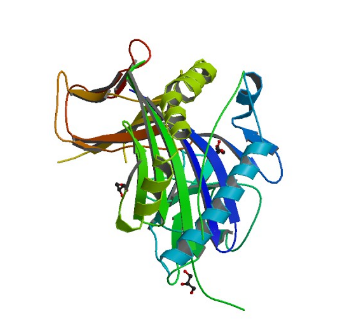
2GTJ: hSH3N domain of ADAP in its reduced form
Image from the RCSB PDB (www.rcsb.org) of PDB ID 2GTJ (Zimmermann, J., Kuhne, R., Sylvester, M., Freund, C.) (2007) Redox-Regulated Conformational Changes in an SH3 Domain Biochemistry 46: 6971-6977
2GTO: hSH3N domain of ADAP in its oxidized form
Image from the RCSB PDB (www.rcsb.org) of PDB ID 2GTO (Zimmermann, J., Kuhne, R., Sylvester, M., Freund, C.) (2007) Redox-Regulated Conformational Changes in an SH3 Domain Biochemistry 46: 6971-6977
3FMA: Smy2 GYF domain in complex with proline-rich peptide
Image from the RCSB PDB (www.rcsb.org) of PDB ID 3FMA (Ash, M.R., Faelber, K., Kosslick, D., Albert, G., Roske, Y., Kofler, M., Schuemann, M., Krause, E., Freund, C.) (2009) SMY2-type GYF domain recognition in mRNA surveillance complexes to be pubished
3K3V: Smy2 GYF domain (S. cerevisiae)
Image from the RCSB PDB (www.rcsb.org) of PDB ID 3K3V (Ash, M.R., Faelber, K., Kosslick, D., Albert, G., Roske, Y., Freund, C.) (2010) SMY2-type GYF domain recognition in mRNA surveillance complexes to be published
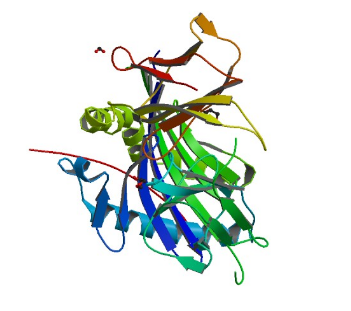
3PDO: HLA-DR1 with CLIP (102-120)
Image from the RCSB PDB (www.rcsb.org) of PDB ID 3PDO (Gunther, S., Schlundt, A., Sticht, J., Roske, Y., Heinemann, U., Wiesmuller, K.H., Jung, G., Falk, K., Rotzschke, O., Freund, C.) (2010) Bidirectional binding of invariant chain peptides to an MHC class II molecule. Proc.Natl.Acad.Sci.USA 107: 22219-22224
3PGC: HLA-DR1 with CLIP (106-120) in its flipped orientation
Image from the RCSB PDB (www.rcsb.org) of PDB ID 3PGC (Gunther, S., Schlundt, A., Sticht, J., Roske, Y., Heinemann, U., Wiesmuller, K.H., Jung, G., Falk, K., Rotzschke, O., Freund, C.) (2010) Bidirectional binding of invariant chain peptides to an MHC class II molecule. Proc.Natl.Acad.Sci.USA 107: 22219-22224
3PGD: HLA-DR1 with CLIP (106-120) in its canonical orientation
Image from the RCSB PDB (www.rcsb.org) of PDB ID 3PGD (Gunther, S., Schlundt, A., Sticht, J., Roske, Y., Heinemann, U., Wiesmuller, K.H., Jung, G., Falk, K., Rotzschke, O., Freund, C.) (2010) Bidirectional binding of invariant chain peptides to an MHC class II molecule. Proc.Natl.Acad.Sci.USA 107: 22219-22224
4AEN: HLA-DR1 with covalently linked CLIP (106-120) in its flipped orientation
Image from the RCSB PDB (www.rcsb.org) of PDB ID 4AEN (Schlundt, A., Gunther, S., Sticht, J., Wieczorek, M., Roske, Y., Heinemann, U., Freund, C.) (2012) Peptide Linkage to the Alpha-Subunit of Mhcii Creates a Stably Inverted Antigen Presentation Complex. J.Mol.Biol. 423: 294
4AH2: HLA-DR1 with covalently linked CLIP (106-120) in its canonical orientation
Image from the RCSB PDB (www.rcsb.org) of PDB ID 4AH2 (Schlundt, A., Gunther, S., Sticht, J., Wieczorek, M., Roske, Y., Heinemann, U., Freund, C.) (2012) Peptide Linkage to the Alpha-Subunit of Mhcii Creates a Stably Inverted Antigen Presentation Complex. J.Mol.Biol. 423: 294
4X5X: HLA-DR1 mutant bN82A with covalently linked CLIP (106-120, M107W)
Image from the RCSB PDB (www.rcsb.org) of PDB ID 4X5X (Wieczorek, M., Sticht, J., Stolzenberg, S., Gunther, S., Wehmeyer, C., El Habre, Z., Alvaro-Benito, M., Noe, F., Freund, C.) (2016) MHC class II complexes sample intermediate states along the peptide exchange pathway. Nature Communications 7: 13224-13224