ON-TARGET Study

Non-interventional study on Therapeutic Drug Monitoring (TDM) in patients with renal cell carcinoma and on the feasibility of using Volumetric Absorptive Microsampling (VAMS) for sample collection (ON-TARGET)
News
- Find a current overview of the ON-TARGET study "One dose to treat them all? – Therapeutisches Drug Monitoring zur Dosisoptimierung in der oralen Antitumortherapie" in the German journal Best Practice Onkologie (Link)
- Publication of the ON-TARGET Protocol paper "Developing a nationwide infrastructure for Therapeutic Drug Monitoring of targeted oral anticancer drugs: The ON-TARGET study protocol" in the journal Cancers (Link)
- ON-TARGET wins the Lesmüller Poster Award for the best poster presentation in the category "Clinical Pharmacy" at the annual conference of the German Pharmaceutical Society e.V. (Link)
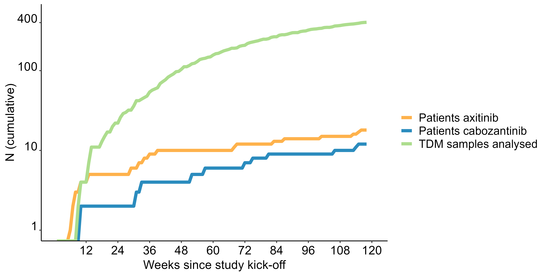
Number of included patients since study kick-off
Background
Treatment with innovative targeted oral drugs in tablet form has greatly improved the prognosis of many tumour types and increased the quality of life compared to conventional chemotherapy.
However, these advantages are often offset by complex intake regimens, a high interaction potential, and high pharmacokinetic variability.
This means that despite taking the same doses, large differences can occur in the drug concentrations observed in individual patients.
Since all patients currently receive the same dose, the resulting drug concentrations are often either too high or too low, which can result in increased adverse drug reactions or reduced efficacy.

Figure modified from Groenland et al. (2019)
Study goal
Routine determination of drug concentrations in the blood as a therapeutic standard offers the possibility to compare measured drug concentrations with therapeutic target ranges within the framework of therapeutic drug monitoring (TDM).
Using TDM, drug doses can be tailored to patients based on the measured drug concentrations. For the drugs investigated in this study, the focus is primarily on adverse drug reactions. By measuring the drug concentrations in the blood, the probability of severe adverse drug reactions can be estimated at an early stage and the treatment can be adjusted accordingly. For this purpose, drug concentration threshold values that have been associated with an increased occurrence of adverse drug reactions in clinical studies are used. At the same time, it will be investigated, whether the routine measurement of the concentrations of disease-specific endogenous proteins, so-called biomarkers, allows an early estimation of the frequency and severity of adverse drug reactions.
Another study objective is the investigation of the practicability of Volumetric Absorptive MicroSampling (VAMS) for TDM sample collection. Similar to routine blood glucose monitoring, only a few drops of blood from the fingertip are needed for the minimally invasive VAMS technology to measure drug concentrations. Due to the uncomplicated sample collection and the possibility to send the VAMS blood samples to the laboratories via normal post, patients will be offered to collect their blood samples at home in the second study phase.
Overall, this study aims to establish the basis for developing a nationwide infrastructure for the implementation of routine TDM in patients undergoing oral anticancer therapy.
Study workflow
The treating physician decides regarding:
- the treatment, i.e. prescription and dosing of axitinib and cabozantinib
- Need for performing routine TDM during treatment with axitinib and cabozantinib
- If informed consent has been given: Inclusion of patients into the ON-TARGET study (independent from the performance of a TDM)
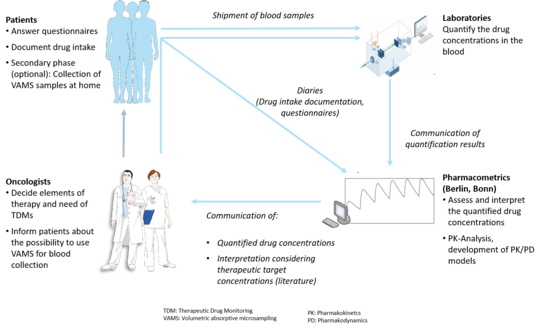
Figure created using Servier Smart Medical Art
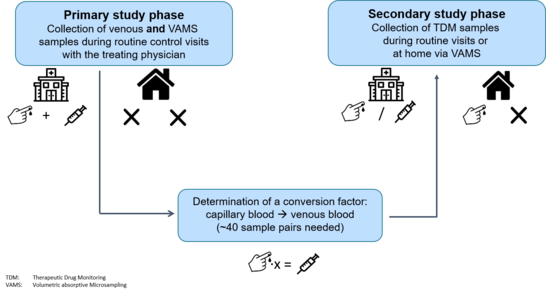
Primary and secondary phase
Primary phase:
- Collection of venous and capillary blood (using VAMS)
- Determination of a conversion factor (capillary -> venous)
- 2 sample pairs per patient targeted
Secondary phase:
- Collection of TDM samples (venous or capillary) during routine visits with the treating physician or at home (capillary only)