Research
1. Molecular patterns and interactions in mucus-like hydrogel systems elucidated by EPR spectroscopy.
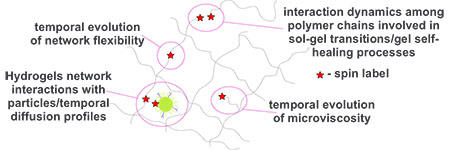
Understanding the intricate structure and dynamic behavior of mucus is crucial for unraveling its multifaceted roles in biological systems. Mucus, with its complex composition and viscoelastic properties, plays pivotal roles in protecting and lubricating epithelial surfaces, facilitating nutrient absorption, and serving as a barrier against pathogens. To comprehensively elucidate the functionality of mucus and its implications for health and disease, it is imperative to study the basics of formation, interactions and diffusion patterns occurring within mucus systems.
The project is focused on the development of the synthetic ways to produce different polymer/cross-linker pairs and to attach different spin labels to the polymers/particles in the site directed manner. We elucidate a molecular-level understanding of the kinetics and interactions in the studied mucus-like hydrogel systems. We use EPR spectroscopy to investigate the patterns of the sol-gel phase transition temporal evolution. The sol-gel process kinetics will be studied with in situ room-temperature continuous wave (CW) EPR in aqueous solutions, that allows one to monitor the changes occurring in the particular polymer/cross-linker mixture properties in real time. We will also use EPR to investigate the interactions of the synthetic mucus-like hydrogels, with different species (nanoparticles/dendritic polymers/proteins/drugs) as they diffuse through the hydrogel. This could potentially provide the information about the temporal diffusion profiles, as well as about possible interactions of certain groups in the polymer network with pathogens, which might be the reason for the mucus defense mechanism. Finally, we will investigate the properties, interactions and diffusion patterns within native mucus systems.
Topics:
1. Investigation of the temporal evolution of the sol-gel phase transition in mucus-mimicking synthetic hydrogels using variable length paramagnetic labels.
You will learn:
EPR spectroscopy, polymer filtration, hydrogels formation, spin labeling, writing and presentation skills, scientific data evaluation skills.
2. Monitoring interactions of natural mucins and mucin-like synthetic polymers with particles/drugs/biomacromolecules/pathogens.
You will learn:
EPR spectroscopy, polymers/proteins spin labeling, bacteria growth and labeling, z-potential and DLS, writing and presentation skills, scientific data evaluation skills.
2. Pepsin stability and activity in water, saturated with CO2 under high pressure.
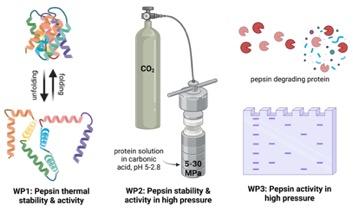
Water becomes acidic upon contact with carbon dioxide due to the formation and dissociation of carbonic acid. As the pressure of CO2 in water increases to 30 MPa, the pH can drop to around 2.8. Despite the clear advantages of this biocompatible and environmentally friendly solvent, there are very few studies in literature on the effects of such high-pressure biphasic media on biomacromolecules. It is known that high pressure CO2can alter the functionality of some proteins, mostly due to the low pH. For example, peach proteins have been reported to aggregate in the solution under high pressure in a time-dependent manner. Although, there is significant interest in performing biocatalysis in “green” solvents, such as water, supercritical CO2, or water saturated with high-pressure CO2. Enzymatic activity in such systems can depend on factors like pressure, pH, formation of carbamates between CO2 and lysine residues and the depressurization rate.
Pepsin is typically found in the stomachs of mammals and is optimized to function in the acidic environment as an integral part of the digestion process. It is a proteolytic enzyme that catalyzes the hydrolysis of peptide bonds in most proteins, DNA, and polysaccharides at pH values below 5. Pepsin is known to adopt different conformations and exhibit varying enzymatic activity depending on the solution’s pH, with at least two distinct conformations at pH values from 7 to 3. This pH range is achievable by adjusting the pressure in the reactor. The best activity is observed at the pH of around 3.5. Therefore, this enzyme is a perfect candidate to act in the carbonic acid solutions. The biocompatible, self-neutralizing nature of this solvent allow one to benefit from the “green” chemistry principles. Structurally, pepsin is a small (35 kDa) globular protein with 327 amino acid residues, and its secondary structure consists mostly of pleated sheets. Previous studies on the thermal unfolding of pepsin using calorimetry (which requires a large amount of material) and circular dichroism (CD) spectroscopy (which necessitates salt-free solutions) have yielded conflicting results. Moreover, the pH was not varied systematically. Therefore, the innovative nano dynamic scanning fluorimetry (nanoDSF) equipment available at SupraFAB will help to address this research gap and provide insights into pepsin’s stability and aggregation behavior under different conditions.
Topics:
1. Pepsin thermal stability, secondary structure, aggregation behavior and activity in biomacromolecules digestion depending on pH at normal atmospheric pressure.
You will learn:
nanoDSF, CD spectroscopy, DLS, SDS-PAGE, writing and presentation skills, scientific data evaluation skills.
2. Effect of CO2 under high pressure and carbonic acid formation on the structure, stability and activity of pepsin protein
You will learn:
work with CO2 solutions under high pressure (300 atm), nanoDSF, CD spectroscopy, DLS, SDS-PAGE, writing and presentation skills, scientific data evaluation skills.
3. Polyelectrolyte protein interactions
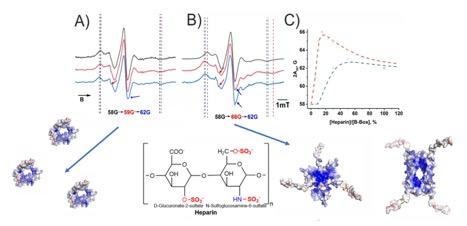
In light of the strong correlation between structure and function of proteins, we aim to elucidate the impact of the interaction between proteins and glycosaminoglycans (GAGs) on protein structure by combining various spectroscopic techniques including circular dichroism (CD), fluorescence, IR, or electron paramagnetic resonance (EPR) spectroscopy, which allows us to study protein-GAG interactions from multiple perspectives. Previously, we focused on the interaction of HMGB1 and its individual domains with heparin as a prototypical sulfated glycan. To this end, it was found using dynamic light scattering (DLS) that the B-box domain of the HMGB1 protein tend to from multimeric self-complexes. This tendency which is also found for the full-length protein but not for the A-box domain might play a role in the formation of HMGB1 bio-condensates in the cellular environment reported in literature. Importantly, the secondary structure as probed by different spectroscopic techniques was shown to be preserved in the multimeric complexes. The addition of Heparin not only perturbs the protein structure but also weakens the interaction with protein monomers as compared to protein self-complexes. This can be rationalized by modeling several oligomeric structures using AlphaFold as these simulations suggest increased electrostatic interactions due to the formation of electrostatic patches on the surface of the oligomers. Additional studies focus on the structural properties of the A-box domain where apart from EPR also fluorescence, CD and FT-IR spectroscopy were used to provide structural insights e.g. with respect to the impact of the redox state i.e. the presence of a disulfide bridge.
Next, we will employ the spectroscopic toolbox mentioned above to still focus on the structural implications of the interaction between proteins and polyelectrolytes but extend the scope to a broader range of glycosaminoglycans (e.g., heparin, keratan sulfate, hyaluronic acid, dendritic polyglycerol sulfate), and also include proteins with intrinsically disordered regions (IDR) (e.g., the C-tail of HMGB1, TFEB, amyloid beta). Here, the role of the IDR for the formation of bio-condensates, as well as the correlation of these interactions with potential conformational changes will be of particular interest.
Topics:
1. Protein/polyelectrolyte and protein/protein interactions: unraveling structural patterns.
You will learn:
EPR, CD, fluorescence spectroscopies, proteins spin labeling, nanoDSF, DLS, AlphaFold modelling, writing and presentation skills, scientific data evaluation skills.
4. Cationic polymers solubility and materials synthesis in carbonic acid solutions under high CO2pressure.
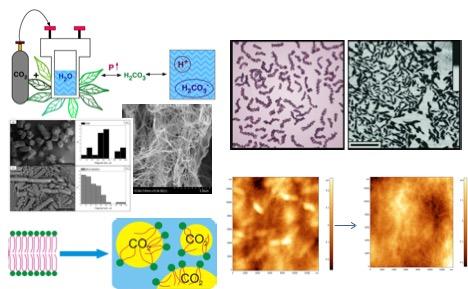
Nowadays, the general vector of polymer science development is pointed at respecting the environment, at refusing to use potentially allergenic substances in the biomedical field, at meeting the requirements of extra purity in catalysis applications, and at imparting new functional properties to composites. In this regard, studies in biocompatible solvents that do not require subsequent neutralization, such as water saturated with CO2under high pressure (solution of carbonic acid), as well as in pure supercritical (SC) CO2, that can impart new properties with advantages to substances modified in such media, look promising.
The developments of our team in this area include the dissolution of cationic polymers (including chitosan) in carbonic acid under high CO2 pressure without the use of traditional acids and the creation of sponges (with/or without cross-linking agents such as genipin, or metal nanoparticles), composites with other polymers (for example, with bacterial cellulose), as well as complexes with metal nanoparticles (Ag, Au, Pt and Cu) based on these solutions. These composites can be in the form of hydrogels, dry sponges, films and capsules. Further development of these new materials can be achieved through supercritical impregnation of drugs into them. Moreover, we have shown that after treatment in SC CO2, or in a solution of carbonic acid under high pressure CO2, the size of drug crystallites can be significantly reduced, which can improve their bioavailability. Thus, following the paradigm of using media containing CO2 under high pressure as the main "green" solvents we have created many different materials with improved mechanical properties, increased surface functionality, antimicrobial properties, reusable catalyst properties, as well as materials suitable for transdermal delivery of hormones (for example, b-estradiol).
Topics:
1. Formation of coatings from dendritic polyglycerol amine in carbonic acid under high CO2 pressure on substrates for neurons growth.
You will learn:
work with CO2 solutions under high pressure (300 atm), AFM, DLS, SEM, writing and presentation skills, scientific data evaluation skills.
2. Lignin-based hydrogel formation in carbonic acid solutions under high CO2 pressure for biomedical applications.
You will learn:
work with CO2 solutions under high pressure (300 atm), lyophilization, supercritical impregnation, AFM, DLS, SEM, writing and presentation skills, scientific data evaluation skills.
