Gas-Phase Spectroscopy of Peptides and Proteins
In cooperation with the groups of Gert von Helden and Carsten Baldauf from the Fritz Haber Institute (FHI) of the Max Planck Society, we investigate amino acids, peptides and proteins in the gas phase using infrared multiple photon dissociation (IRMPD) spectroscopy.
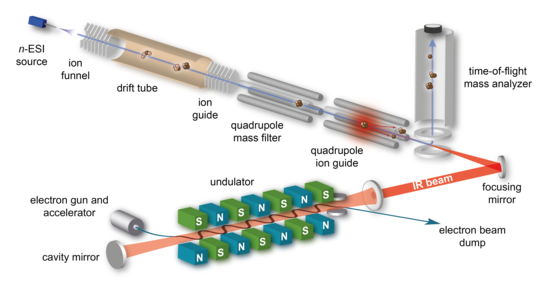
In the condensed phase, IR spectroscopy is a well-established tool to analyze the structure and dynamics of proteins and their assemblies. This is based on the fact that the position of the characteristic amide I and amide II vibrational bands provides a direct measure of the molecules secondary structure. In the absence of solvent, IR spectra can also be measured using action spectroscopy techniques. The absence of reference values for species with defined conformation as well as the typical peak broadening observed for larger molecules, however, so far restricted the applicability of the method to small peptides with usually no more than ten residues. Here, we are aiming to extend the effective size-range of the method to folded proteins and protein complexes using conditions that are specifically optimized for retention of the molecules conformation in the gas phase. Tunable infrared radiation for these experiments is provided by the FHI free electron laser.
Related Publications
- Side-chain effects on the structures of protonated amino acid dimers: A gas-phase infrared spectroscopy study
Seo, J.; Hoffmann, W.; Malerz, S.; Warnke, S.; Bowers, M. T.; Pagel, K.; von Helden, G.; Int. J. Mass. Spectrom. 2018, 429, 115–120.
- Structure and infrared spectrum of the homochiral serine octamer chloride adduct
Seo, J.; Warnke, S.; Pagel, K.; Bowers, M. T.; von Helden, G.; Nature Chem. 2017, 9, 1263–1268.
- From compact to string – the role of secondary and tertiary structure in charge-induced unzipping of gas-phase proteins
Warnke, S.; Hoffmann, W.; Seo, J.; De Genst, E.; von Helden, G.; Pagel, K.; J. Am. Soc. Mass Spectrom. 2017, 28, 638–646. - Retention of native protein structures in the absence of solvent: A coupled ion mobility and spectroscopic study
Seo, J.; Hoffmann, W.; Warnke, S.; Bowers, M. T.; Pagel, K.; von Helden, G.; Angew. Chem. Int. Ed. 2016, 55, 14173–14176. - Protomers of benzocaine: Solvent and permittivity dependence
Warnke, S.; Seo, J.; Boschmans, J.; Sobott, F.; Scrivens, J. H.; Bleiholder, C.; Bowers, M. T.; Gewinner, S.; Schöllkopf, W.; Pagel, K.*; von Helden, G.*, J. Am. Chem. Soc. 2015, 137, 4236–4242 - Photodissociation of conformer-selected ubiquitin ions reveals site-specific cis/trans isomerization of proline peptide bonds
Warnke, S.; Baldauf, C.; Bowers, M.T.; Pagel, K.; von Helden, G.; J. Am. Chem. Soc. 2014, 136, 10308–10314.
- How cations change peptide structure
Baldauf, C.; Pagel, K.; Warnke, S.; von Helden, G.; Koksch, B.; Blum, V.; Scheffler, M.; Chem. Eur. J. 2013, 19, 11224–11234. -
Gas-phase IR spectra of intact α-helical coiled coil protein complexes
Pagel, K.; Kupser, P.; Bierau, F.; Polfer, N.C.; Steill, J.D.; Oomens, J.; Meijer, G.; Koksch, B.; von Helden, G.; Int. J. Mass Spectrom. 2009, 283, 161–168.




