New Publication in Organic Letters
Direct Experimental Characterization of the Ferrier Glycosyl Cation in the Gas Phase
News from Nov 04, 2020
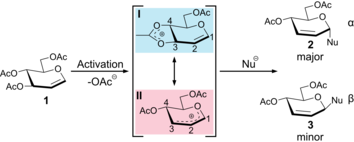
Fig.1: Ferrier Rearrangement Reaction
In 1917, Emil Fischer reported a novel class of unsaturated sugars, the so-called glycals. It took almost 50 years before their rich chemistry was exploited by Robert J. Ferrier, who also perceived their pharmaceutical potential. He described the reaction of an acylated glycal that is attacked by a nucleophile leading to the cleavage of a C3-acyl protecting group. Subsequently, the nucleophile attacks at the C1 position followed by an allylic shift of the double bond. The reaction is now known as the Ferrier rearrangement. Due to its short lifetime, the exact structure of the cationic intermediate, the Ferrier cation, could not be determined until now. Two structural motifs have been postulated: an oxocarbenium-type species, where the positive charge is delocalized within the pyranose ring, and a dioxolenium-type species, where the positive charge at the C3 atom is stabilized by participation of the neighboring C4-acetyl group. We were able to generate Ferrier cations of acetylated glucal and galactal in the gas phase and probe their vibrational modes using cryogenic infrared spectroscopy. The spectral signature clearly shows that the underlying structural motif is a dioxolenium-type structure. These findings provide novel insights into the mechanistic understanding of the Ferrier rearrangement reaction, which is particularly important for glycosynthesis.
go to article: DOI: 10.1021/acs.orglett.0c03301.




