New Publication in Journal of the American Chemical Society
Unveiling Glycerolipid Fragmentation by Cryogenic Infrared Spectroscopy
News from Sep 02, 2021
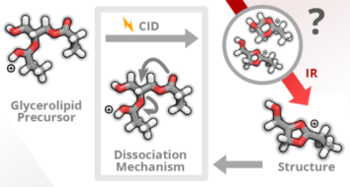
Tandem mass spectrometry is routinely employed for structure elucidation of lipids; however, the interpretation of fragment spectra is often hampered by poor understanding of the underlying dissociation mechanisms. In this work, we investigate neutral headgroup loss from protonated glycerolipids using cryogenic gas-phase infrared (IR) spectroscopy in combination with computational chemistry. We show that glycerolipids undergo a ring closure reaction in the gas phase and form five-membered dioxolane rings. Furthermore, the cyclic structure of an intermediate fragment occurring in the phosphatidylcholine fragmentation pathway was spectroscopically confirmed. Overall, the results contribute substantially to the understanding of glycerolipid fragmentation and showcase the value of vibrational ion spectroscopy to mechanistically elucidate crucial fragmentation pathways in lipidomics.