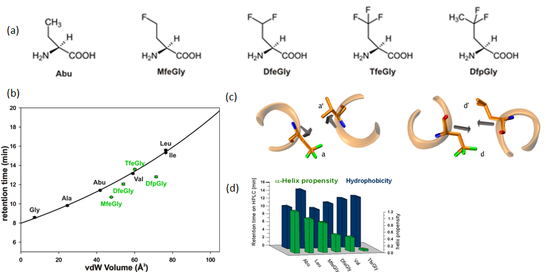
Properties of side chain fluorinated amino acids
Analogues of (S)-2-aminobutyric acid (Abu) with increasing fluorine content have been extensively characterized (Samsonov, Salwiczek, et al., J. Phys. Chem. B, 2009). Interestingly, whereas a single carbon-fluorine bond in the side chain of Abu decreases the hydrophobicity of the residue, a significant and additive increase in hydrophobicity is observed with the introduction of further carbon-fluorine bonds. Thus, although MfeGly is larger in volume than Abu, it is less hydrophobic. DfeGly is also less hydrophobic than its hydrocarbon/fluorocarbon surface area would suggest. DfpGly, which is very close in size to leucine, is even more polar than the much smaller valine.
Figure 3. (a) Chemical structure of Abu-analogues with increasing fluorine content . (b) Correlation of retention time and solvent accessible surface area of amino acids of side chain fluorinated amino acids. (c) Packing of a and d positions in a parallel coiled coil dimer. (d) Bar chart depicting α-helix propensity vs. hydrophobicity.