Research statement
Two-dimensional nanomaterials and two-dimensional polymers:
Low-dimensional nanomaterials exhibiting unique physicochemical properties have recently emerged as promising tools for different applications ranging from energy storage to cancer diagnosis and treatment. Among this class of materials, two-dimensional nanomaterials including graphene, metal dichalcogenides and black phosphorous have attracted much attention due to their huge surface area and prominent optical, electrical, photothermal, and photodynamic properties along with outstanding drug loading capacity and fast cellular uptake. Two-dimensional polymers (2D polymers), on the other hand, are single-monomer-thick two-dimensional nanomaterials (2D nanomaterials) with defined and covalently linked repeating unites. Physicochemical properties of both 2D nanomaterials and 2D polymers depend strongly on their surface chemistry, which is defined by a combination of parameters including functionality, charge, heteroatom doping, defects, edges and number of layers. All of these factors must be standardized or clearly defined to shed more light on the relation between molecular structure and physicochemical properties of 2D nanomaterials and 2D polymers and guide further development for different applications.
My research interests are primarily focused towards two-dimensional polymers and two-dimensional nanomaterials and deepening our understanding of the mechanism of their synthesis as well as investigation of their physicochemical and biological properties. I am seeking for straightforward polymerization methods for the preparation of a broad family of 2D polymers for a wide range of biomedical, energy and optoelectronic applications.
To achieve these goals we have focused on three strategies:
i) Synthesis of polymer-coated two-dimensional nanomaterials with defined functionality: investigation of their interactions at nano-biointerfaces
ii) Synthesis of two-dimensional polyols using graphene platform: New systems for therapeutic delivery and molecular recognition at biointerfaces
iii) Metal-assisted synthesis of two-dimensional polymers: their optoelectronic applications
i) Synthesis of polymer-coated two-dimensional nanomaterials with defined functionality: investigation of their interactions at nano-biointerfaces
Over the past several years my group has developed a new nondestructive covalent functionalization method for graphene and carbon nanotubes through which we have been able to synthesized photoswitchable nanodevices for optoelectronic applications. In spite of other covalent functionalizations, the π-conjugated system and conductivity of graphene are preserved and even improved by our method (Figures 1a,b). The performance of this method for the construction of 2D platforms for sensor, energy storage and catalyst applications can be investigated through joint projects within the future collaborations.
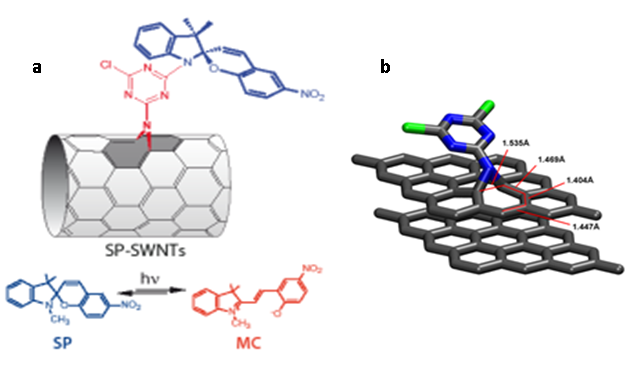
Figure 1. a) Spiropyran-nanotube molecules (SP-SWNTs) switch for photoactivated doping of carbon nanotubes. Spiropyran (SP) is covalently grafted via triazine anchors onto the nanotube. The molecule undergoes a transition into the open merocyanine (MC) form upon UV irradiation. b) Graphene functionalized by our covalent nondestructive method. Atomic structure contained in unit cell with marked bond lengths around the triazine addition site. Opening of bridge bond in the functionalized site results in rehybridization of carbon atoms of graphene. The rehybridization leads to covalently functionalized graphene sheets with integrated π-conjugated system.
Taking advantage of this method and stepwise post-functionalizations, polyglycerol-coated graphene sheets with defined functionalities were synthesized and they were used for pathogen interactions or hyperthermia surmounting of multidrug resistance (Figures 2a,b).
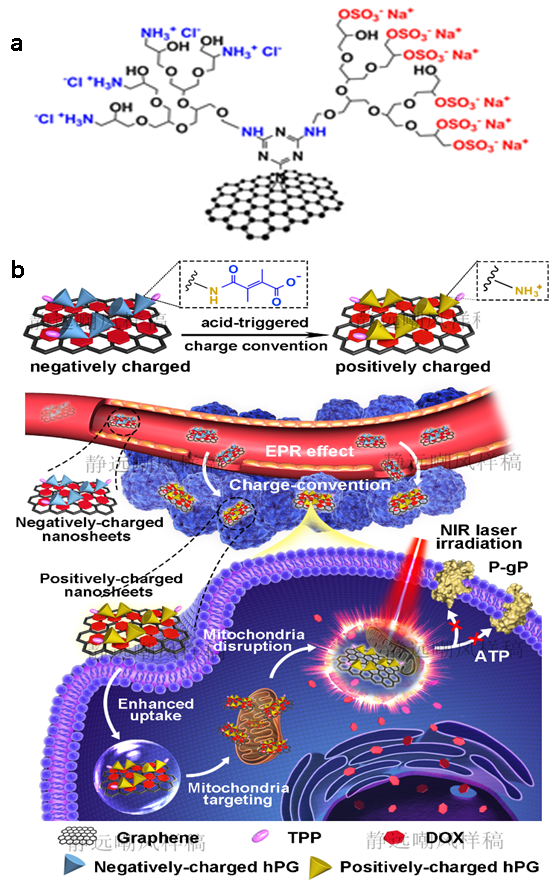
Figure 2. a) Zwitterionic graphene sheets synthesized by stepwise and controlled functionalization method. Nucleophilic substitution of the first chlorine atom of dichloro-triazine groups, wgich are conjugated onto the surface of graphene, resulted in polyglycerol-functionalized graphene sheet. This compound could be subsequently converted to its sulfated analog by using a sulfur trioxide/pyridine complex. Nucleophilic substitution of the second chlorine atom of triazine groups by polyglycerolamine led to zwitterionic graphene sheets. b) Surface charge conversion of a polyglycerol-functionalized nanographene sheets after permeation into tumor tissue. Negatively charged graphene sheets change to positive sheets in tumor sites and positively charged graphene sheets uptake by cells quickly (top). Hyperthermia surmounting of multiple drug resistance by functionalized graphene sheets. After, charge-mediated cellular internalization, graphene sheets accumulate into the mitochondria by targeting ligands. Mitochondrial dysfunction and accelerated drug release through hyperthermia result in MDR suppression and efficient chemotherapy.
The non-biodegradability of graphene and related health risks, however, are major concerns for the in vivo applications of polyglycerol-coated graphene sheets. Therefore, one of our future plans is to overcome this problem by two methods:
In the first method we will conjugate a combination of glucose peroxidase and myeloperoxidase on the surface of graphene sheets to create a 2D nanomaterial with self-degradation property in the physiological conditions (Figure 3a).
The second method, which is the elimination of graphene from the polyglycerol-coated graphene sheets, will forward us to the next motif:
ii) Synthesis of two-dimensional polyols using graphene platform: New systems for therapeutic delivery and molecular recognition at biointerfaces
One of our current and future plans is to construct highly functional and graphene-free two-dimensional polyols using graphene platforms. We use covalent and noncovalent methods to construct these polymers. In covalent method, polyglycerol branches are conjugated to the surface of graphene by pH cleavable linkers. Then, these branches are crosslinked side by side to obtain a two-dimensional polyglycerol network on the surface of graphene platform. Finally, the two-dimensional polyglycerol will be separated from the graphene platform by acidification and breaking the pH cleavable linkers (Figure 3b). Two-dimensional polyglycerols with several nanometer thickness are water soluble and can be studied in terms of their surface and interface chemistry, and also their interactions with living cells.
In the noncovalent method, supramolecular interactions between graphene platform and monomers are driving forces for the production of 2D polyols. For example, functionalized cyclodextrins are loaded on the surface of graphene by supramolecular interactions and then they are linked side by side to produce a two-dimensional polycylodextrin with several micrometer lateral size and one nanometer thickness (Figure 3c).
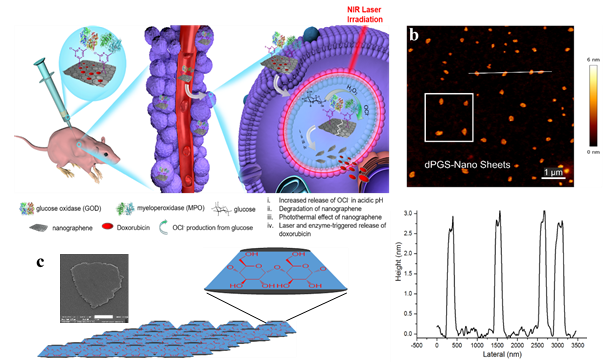
Figure 3. a)Schematic representation of the application of graphene platforms with self-degradation property for the tumor therapy. Glucose oxidase (GOD) and myeloperoxidase (MPO) are conjugated on the surface of graphene by stepwise reactions. Also doxorubicin is loaded on the surface of graphene to make a biodegradable anticancer drug delivery system. Hydrogen peroxide, which is produced by GOD-mediated glucose oxidation, is converted to hypochlorite. This reagent (hypochlorite) plays multiple roles in tumor therapy: it breakdowns graphene sheets to small pieces and decreases their health hazards. It also increases the anticancer effect of drug delivery system by its own therapeutic effect and accelerates the release of loaded doxorubicin. Laser irradiation improves the rate of release of doxorubicin and induces a photothermal effect. b) AFM image of the two-dimensional polyglycerol sheets synthesized by graphene platform (top) and height profile of four labled sheets (average height and lateral sizes are 3 nm and 250 nm, respectively). c) Schematic representation and SEM image of two-dimensional polycyclodextrins.
The supramolecular interactions (host-guest) between small molecules and two-dimensional polycylodextrins is an interdisciplinary topic for the living cell interactions as well as therapeutic delivery and molecular recognition.
