Research
Statement of research interests
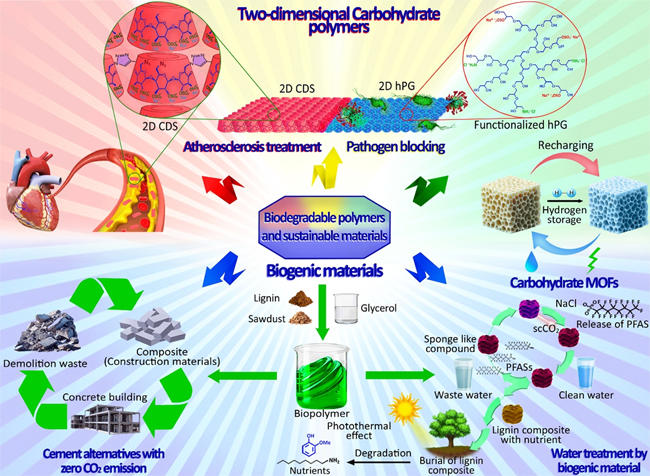
My research focuses on the design, synthesis, and application of functional sustainable materials, emphasizing circular chemistry and the valorization of biogenic waste materials. Specifically, I aim to transform lignin, sawdust, and glycerol, byproducts of the paper, wood, and biodiesel industries, along with construction and demolition waste into high-value, recyclable materials.
Additionally, I am developing biodegradable two-dimensional materials, covalent organic frameworks, and carbohydrate-based metal-organic frameworks for biomedical and environmental applications. By integrating green chemistry principles with expertise in advanced functional materials, my goal is to create innovative platforms that tackle global sustainability challenges, including waste reduction, energy storage, and resource efficiency. Furthermore, my work explores the potential biomedical applications of these materials, particularly in reactive oxygen species (ROS) production, antimicrobial therapies, atherosclerosis treatment, and wound healing.