Stromal cells as tissue organizers in development and regeneration
Stromal cells are an indispensable component of the musculoskeletal system; however they have attracted far less attention than other tissue types. Odd-skipped is a drosophila pair-rule gene involved in embryonic segmentation. In mammals, two odd-skipped paralogs exist, Osr1 and Osr2. Odd-skipped genes encode zinc-finger transcription factors expressed in several organs of the developing embryo. For Osr1, functions in heart and kidney development have been described, while Osr2 has been linked to secondary palate formation and tooth development. We have analysed the expression of Osr1 and Osr2 in the chick limb and found both genes to be highly specific for irregular connective tissue stromal cells. We have shown that Osr1 and Osr2 both promote the differentiation of connective tissue fibroblasts from mesenchymal stromal progenitors, and are potent inhibitors of differentiation along other cell lineages (Stricker et al. 2012).
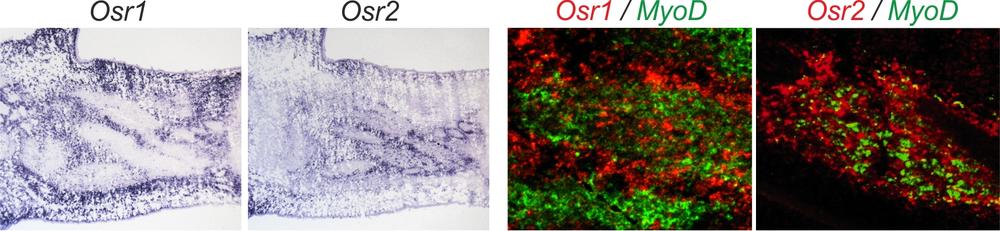
Expression of Osr1 and Osr2: left panel: in-situ hybridisation for Osr1 and Osr2 showing widespread expression of both genes in the chicken limb at Hamburger-Hamilton stage 32. The right panel shows a two-colour ISH for Osr1 or Osr2 together with the myogenic factor MyoD. This shows expression of both Osr genes in chicken muscle connective tissue. Modified from Stricker et al. 2012.
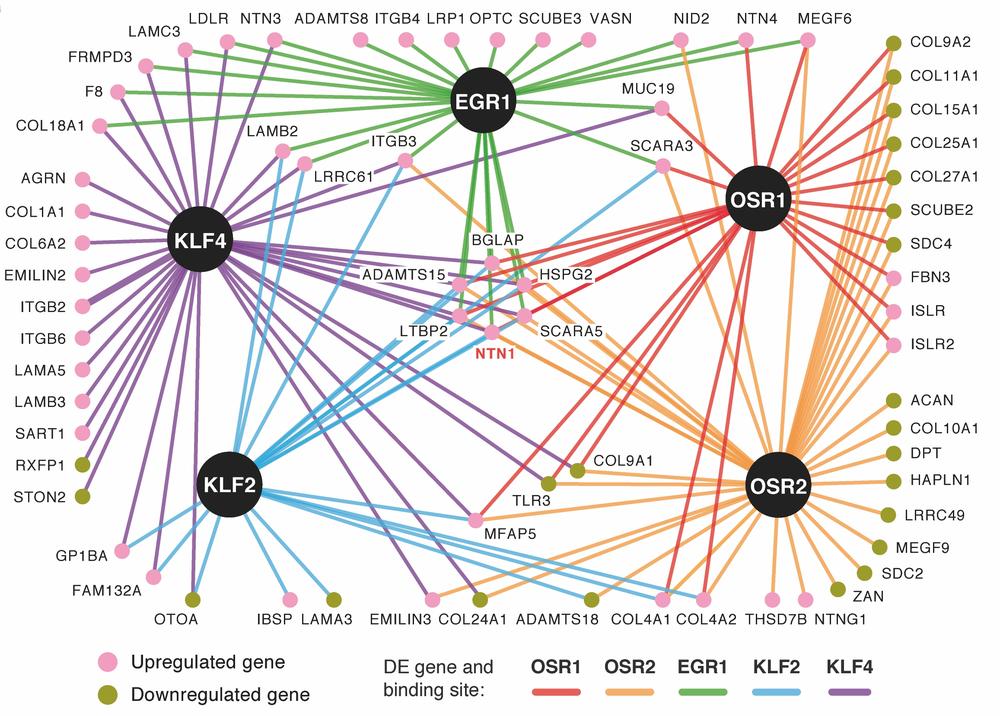
A global analysis of Osr1 target genes in comparison to other connective tissue-expressed transcription factors via combining RNA-Sequencing and ChIP-Sequencing highlighted paracrine signalling molecules as well as extracellular matrix molecules as pivotal downstream targets. Intriguingly, different transcription factors from different connective tissue sub-types showed a remarkable overlap in extracellular matrix-related gene expression, defining a core connective tissue signature. Each transcription factor, on the other hand, regulated specific matrix targets, indicating a transcription factor-based matrix code during limb development, instructive for tissue patterning (Orgeur et al. 2018).
Transcriptional network of five connective tissue-expressed transcription factors reflecting common and individual target genes coding extracellular matrix components. From Orgeur et al. 2018.
Going from chicken to mouse as model system we found a conserved expression pattern of Osr1 in limb bud mesenchymal progenitor cells. Loss of Osr1 function impaired stromal cell / connective tissue-mediated limb muscle patterning. This involved an altered expression of extracellular matrix molecules, which we assume to play a key role in instructing myogenic progenitors and maintaining the myogenic pool.
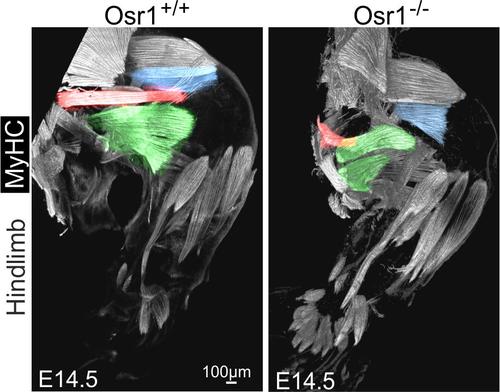
Osr1, expressed in muscle connective tissue, is necessary for correct myogenesis in the mouse limb. Whole hindlimb muscle reconstruction shows defects in several muscles (highlighted in pseudocoloration) in Osr1 mutants. Adapted from (Vallecillo-Garcia et al. 2017).
Furthermore we showed that Osr1-expressing mesenchymal cells in the embryonic limb bud are a developmental source of the so-called fibro-adipogenic progenitors, FAPs, an adult muscle-resident mesenchymal stem cell-like population (Vallecillo-Garcia et al. 2017).
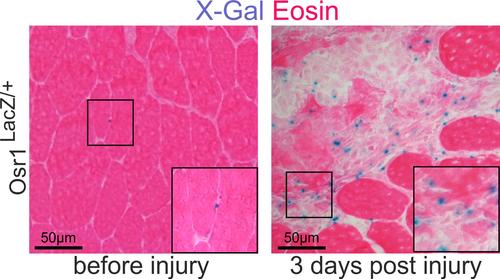
Adult muscle-resident mouse FAPs do not express Osr1, as opposed to their developmental counterparts. Intriguingly, upon acute injury, FAPs re-activate Osr1 expression, making Osr1 a suitable marker for FAPs during muscle injury regeneration (Stumm et al. 2018).
Re-expression of Osr1 in injured muscle shown by LacZ staining from an Osr1-LacZ allele. Adapted from (Stumm et al. 2018)
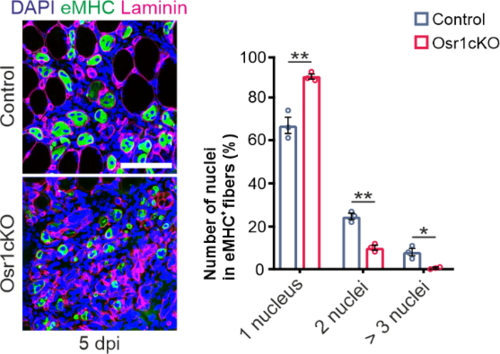
Conditional inactivation of Osr1 during skeletal muscle regeneration resulted in delayed regeneration with persistent fibrosis. Osr1-deficient FAPs switched from a pro-regenerative to a detrimental state actively repressing regenerative myogenesis via TGFbeta1 signaling (Kotsaris et al. 2023).
Immunolabeling for embryonal myosin heavy chain (eMHC) and Laminin shows reduced regenerative myogenesis in Osr1 conditional KO (Osr1cKO) animals. Adapted from (Kotsaris et al. 2023)
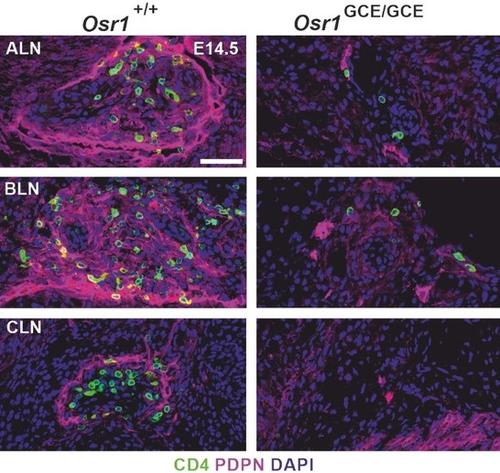
We also asked whether the mechanisms used by mesenchymal cells during muscle patterning might be applicable to other organs. We found that Osr1 is indispensable for lymph node (LN) formation. Osr1 is expressed in so-called lymphoid tissue organizer cells (LTos), which secrete cytokines to attract T-cells, so called lymphoid tissue inducer cells (LTis). Single cell sequencing and immunolabeling allowed us to stratify LTo cells at different anatomical locations, providing insight into regional LTo heterogeneity at the onset of LN formation. Osr1 was required in LTos for autocrine Retinoic Acid signalling, which drives expression of the key cytokine CXCL13. Furthermore, Osr1 was required for lymph vessel assembly at nascent LNs. Lymph vessels produce a second cytokine, CCL21, and only the combined action of both leads to efficient initial LTi attraction. With this, we revised and extended the previous model of LN initiation (Vallecillo-Garcia et al. 2023).
Lack of lymph node induction in Osr1 KO embryos at embryonic day (E) 14.5. LTi cells are labelled green, lymph vessels are labelled red. ALN: alary lymph node, BLN: brachial lymph node, CLN: cervical lymph node. Adapted from (Vallecillo-Garcia et al. 2023).
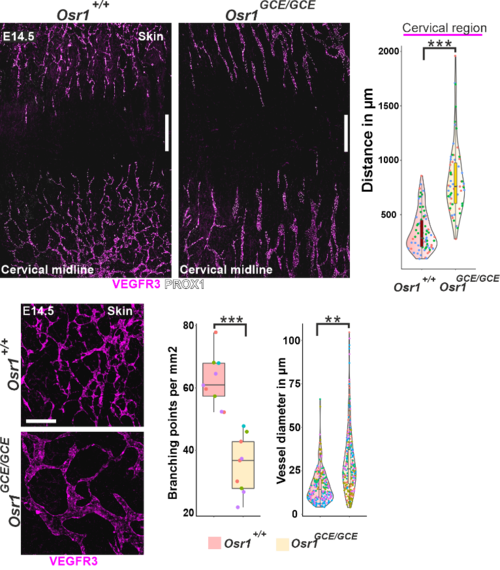
Following up on this, we could recently show that Osr1+ stromal cells play a vital role in the formation of the embryonic lymph vasculature. Lack of Osr1 resulted in decreased migration of lymph endothelial cells towards the dorsal midline, and reduced proliferation of these cells at the migration front. Osr1+ cells acted in a bimodal manner, with extracellular matrix deposited by Osr1+ cells mainly promoting lymph endothelial cell proliferation and secreted factors as CXCL12 driving migration (Vallecillo-Garcia et al. 2024).
Lack of Osr1 causes defects in lymphangiogenesis. Top row: Whole-mount immunofluorescence of E14.5 Osr1+/+ and Osr1GCE/GCE skin samples for VEGFR3 and PROX1; quantification of distance between the tips of the migrating lymphatic vasculature front shown right. Bottom row: Structure of lymph vessels in control and Osr1GCE/GCE embryos; quantification of lymph vessel branching points per mm2 vessel diameter shown right migration. Adapted from (Vallecillo-Garcia et al. 2024).
Collaboration partners:
- Sven Geissler, Berlin Institute of Health
- Petra Knaus, Freie Universität Berlin
- Tim J. Schulz, Deutsches Institut für Ernährungsforschung, Bergholz-Rehbrücke
- Fabien Le Grand, Institut NeuroMyoGene, Lyon, France
- Delphine Duprez, UMR7622, CNRS, Université Pierre et Marie Curie, Paris, France
- David Sassoon, Giovanna Marazzi; ICAN Institute of Cardiometabolism and Nutrition, Paris, France
- Chiara Mozzetta, Dept. of Biology and Biotechnology, Sapienza - University of Rome, Italy