General information
The main goal of our research is to understand the role of the hormone cytokinin in regulating plant growth and development and, more recently, responses to stress. Cytokinin is one of the classical plant hormones, it was discovered during the 1950ties as a factor inducing plant cell division. Chemically, cytokinins are purine derivatives and the main active cytokinins are isopentenyladenine and trans-zeatin. The cytokinin signal is perceived and transmitted by a two-component signaling system. Membrane-located histidine kinases sense cytokinin and transmit the information via phosphotransmitter proteins. These activate B-type response regulator proteins, which are transcription factors realizing the transcriptional output of a cytokinin signal. Our past research has made various contributions to characterize the functions of cytokinins, particularly in the model plant Arabidopsis thaliana. Results have shown that a key activity of cytokinin is to regulate the transition from cell division to cell differentiation. In this way, the hormone regulates quantitatively a number of traits, for example root and stem growth and the size and activity of reproductive meristems, which are relevant for breeding of crop plants. We are exploring the transfer of the knowhow from Arabidopsis to crop plants.
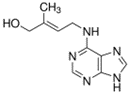
The chemical structure of trans-zeatin,
an important cytokinin.

Arabidopsis wild type (WT) and a cytokinin
receptor mutant.
A second field of study is photoperiod stress, which is led by Anne Cortleven. Photoperiod stress is a novel type of abiotic stress for plants, which is caused by the prolongation of day length. Short-day adapted Arabidopsis plants respond to a prolongation of the light period by several hours with a complex stress response during the following night. Hallmarks of this response is a strong induction of typical stress marker genes, an increase in the concentrations of reactive oxygen species (ROS) and of the stress hormone salicylic acid. Strong photoperiod stress may lead to programmed cell death (PCD) in leaves during the days after the stress. RNA-seq analysis has shown that the response to photoperiod stress resembles the response to pathogen infection.
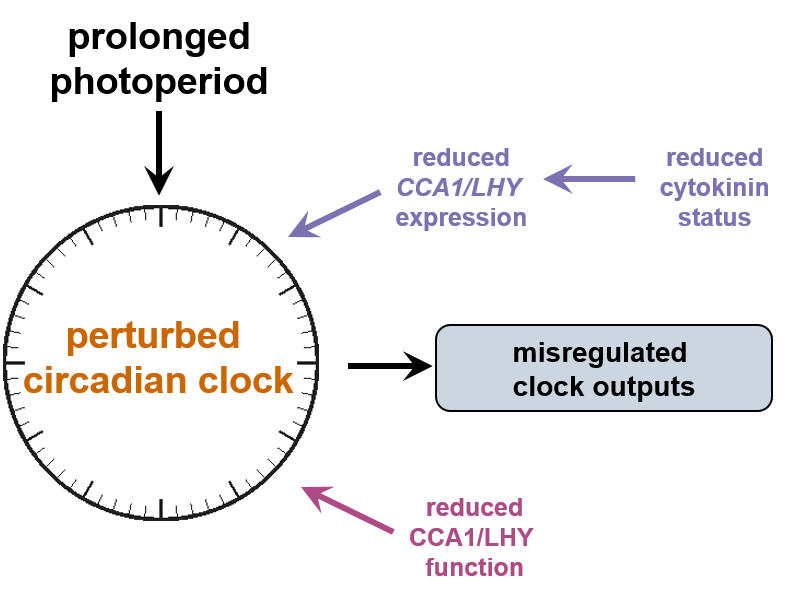
Photoperiod stress is caused
by a prolonged photoperiod.
Werner, T., Schmülling, T. (2009) Cytokinin action in plant development. Curr. Opin. Plant Biol. 12, 527-538. doi:10.1016/j.pbi.2009.07.002.
Cortleven, A., Leuendorf, J.E., Frank, M., Bolt, S., Pezzetta, D., Schmülling, T. (2019) Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environm. 42, 998-1018. https://doi.org/10.1111/pce.13494
Romanov G.A., Schmülling, T. (2022) On the biological activity of cytokinin free bases and their ribosides. Planta 255, 27. https://doi.org/10.1007/s00425-021-03810-1
Romanov, G.A., Lomin, S., Schmülling, T. (2018) Cytokinin signaling: from the ER or from the PM? That is the question! New Phytol. 218, 41-53. (Tansley Review) https://doi.org/10.1111/nph.14991
Brenner, W.G., Schmülling, T. (2015) Summarizing and exploring data of a decade of cytokinin-related transcriptomics. Front. Plant Sci. 6, 29. https://doi.org/10.3389/fpls.2015.00029
Brenner, W.G., Ramireddy, E., Heyl, A., Schmülling, T. (2012) Gene regulation by cytokinin in Arabidopsis. Front. Plant. Sci. 3, 8. https://doi.org/10.3389/fpls.2012.00008
Heyl, A., Riefler, M., Romanov, G.A., Schmülling, T. (2011) Properties, functions and evolution of cytokinin receptors. Eur. J. Cell Biol. 91, 246-256. https://doi:10.1016/j.ejcb.2011.02.009
Werner, T., Köllmer, I., Bartrina, I., Holst, K., Schmülling, T. (2006) New insights into the biology of cytokinin degradation. Plant Biol. (Stuttg.) 8, 371-381. https://doi:10.1055/s-2006-923928.
Heyl, A., Schmülling, T. (2003) Cytokinin signal perception and transduction. Curr. Opin. Plant Biol. 6, 480-488. https://doi:10.1016/s1369-5266(03)00087-6.
Schmülling, T., Werner, T., Riefler, M., Krupková, E., Bartrina y Manns, I. (2003) Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J. Plant Res. 21, 40-49. https://doi:10.1007/s10265-003-0096-4
Nitschke, S., Cortleven, A., Schmülling, T. (2017) Novel stress in plants by altering the photoperiod. Trends in Plant Sci. 22, 913-916. https://doi.org/10.1016/j.tplants.2017.09.005
Roeber, V.M., Bajaj, I., Rohde, M., Schmülling, T., Cortleven, A. (2021) Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 44, 645-664. https://doi.org/10.1111/pce.13948
Roeber, V.M., Schmülling, T., Cortleven, A. (2022) The photoperiod: handling and causing stress in plants. Frontiers in Plant Science 12, 781998. https://doi.org/10.3389/fpls.2021.781988
Current projects
- Regulation of developmental transitions by cytokinin
The role of cytokinin in regulating developmental transitions such as seed germination, juvenile-to adult transition of leaves or the transition to flowering is as yet not well explored. Recently we have shown that cytokinin regulates the juvenile-to-adult transition of leaves through components of the age pathway. Work deciphering the molecular mechanisms of cytokinin action is ongoing. This project is led by Sören Werner. Other projects explore the fine-tuning of seed germination and flowering time by cytokinin (in collaboration with Isabel Bartrina, University of Graz).
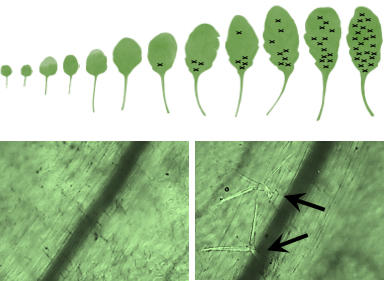
The appearance of trichomes is a hallmark of
the juvenile-to-adult transition in Arabidopsis leaves.
Werner S., Bartrina, I., Schmülling T. (2021) Cytokinin regulates vegetative phase change in Arabidopsis thaliana through the miR172/TOE1-TOE2 module. Nature Communications 12, 5816. https://doi.org/10.1038/s41467-021-26088-z
- Cytokinin action through transcriptional regulation
Great part of the cytokinin signaling output is realized through the altered expression of cytokinin response genes. This is achieved through B-type response regulators (RRBs), which are transcription factors (TFs) of the Myb family. Interaction of RRBs with other TFs contributes to the variability and the specification of the transcriptional output. We have carried out a genome-wide yeast two-hybrid interaction screen of Arabidopsis RRBs with other TFs and are currently exploring the biological significance of some of these interactions. This project is led by Jan Erik Leuendorf.
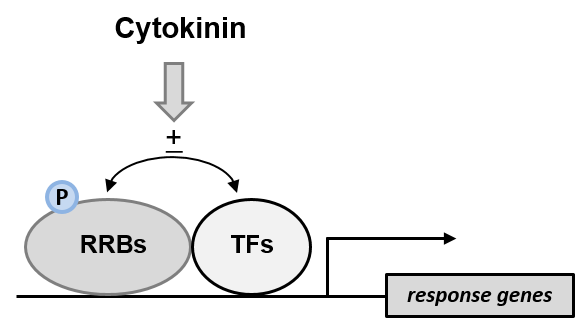
Interaction of different transcription factors
modulates the transcriptional response to cytokinin.
Leuendorf, J.E., Schmülling, T. (2021) Meeting at the DNA: Specifying cytokinin responses by modulating the transcriptional output. Plants 10, 1458. https://doi.org/10.3390/plants10071458
Brenner, W.G., Schmülling, T. (2015) Summarizing and exploring data of a decade of cytokinin-related transcriptomics. Front. Plant Sci. 6, 29. https://doi.org/10.3389/fpls.2015.00029
Brenner, W., Romanov, G., Bürkle, L., Schmülling, T. (2005) Immediate-early and delayed cytokinin response genes of Arabidopsis thaliana identified by genome-wide expression profiling reveal novel cytokinin-sensitive processes and suggest cytokinin action through transcriptional cascades. Plant J. 44, 314-333. https://doi:10.1111/j.1365-313X.2005.02530.x
Heyl, A., Ramireddy, E., Brenner, W.G., Riefler, M., Allemeersch, J., Schmülling, T. (2008) The transcriptional silencer ARR1-SRDX suppresses pleiotropic cytokinin activities in Arabidopsis. Plant Physiol. 147, 1380-1395. https://doi:10.1104/pp.107.115436
Brenner, W.G., Schmülling, T. (2012) Transcript profiling of cytokinin action in Arabidopsis roots and shoots discovers largely similar but also organ-specific responses. BMC Plant Biol. 12, 112. https://doi:10.1186/1471-2229-12-112
Brenner, W.G., Ramireddy, E., Heyl, A., Schmülling, T. (2012) Gene regulation by cytokinin in Arabidopsis. Front. Plant. Sci. 3, 8. https://doi.org/10.3389/fpls.2012.00008
Ramireddy, R., Pfeifer, A., Brenner, W.G., Heyl, A.*, Schmülling, T.* (2013) In planta analysis of a cis-regulatory cytokinin response motif in Arabidopsis and identification of a novel enhancer sequence. Plant Cell Physiol. 54, 1079-1092. https://doi:10.1093/pcp/pct060
- Translational research: Regulation of seed yield by cytokinin
Seed yield is one of the most important traits in breeding of crop plants. We have shown that mutation of specific genes encoding cytokinin-degrading cytokinin oxidases/dehydrogenase (CKX) enzymes causes a substantial increase in seed yield in Arabidopsis. The yield-related traits that are regulated by cytokinin are the size and activity of the shoot apical meristem, the number of flowers and siliques that are formed, the density and number of seeds in siliques, and the duration of flowering time. Together, this underpins the relevance of sink strength in determining yield and identifies CKX genes as promising candidates for practical breeding. Recently, analysis of the orthologous CKX genes of oilseed rape (Brassica napus) has lend support for their potential role in determining seed yield. ckx3 ckx5 mutants showed more active inflorescence meristems and produced >50% more flowers with gynoecia containing more ovules on the main stem. We are currently exploring the individual contributions of B. napus CKX gene family members to regulate yield traits in oilseed rape. This project is led by Ireen Schwarz.
Recently, we have extended our analysis to barley (Hordeum vulgare), which is an important European cereal. This work is led by Jan Erik Leuendorf and carried out in collaboration with Joachim Kumlehn and Robert Hoffie, IPK Gatersleben.

An inflorescence of oilseed rape.
Bartrina, I., Otto, E., Strnad, M., Werner, T., Schmülling, T. (2011) Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23, 69-80. https://doi:10.1105/tpc.110.079079.
Bartrina, I., Jensen, H., Novak, O., Strnad, M., Werner, T., Schmülling, T. (2017) Gain-of-function mutants of the cytokinin receptors AHK2 and AHK3 regulate plant organ size, flowering time and plant longevity. Plant Physiol. 173, 1783-1797. https://doi:10.1104/pp.16.01903.
Schwarz, I., Otto, E., Marie-Therese Scheirlinck, M.-T., Bartrina, I. Schmidt, R., Schmülling, T. (2020) Cytokinin regulates the activity of the inflorescence meristem and components of seed yield in oilseed rape. J. Exp. Bot. 71, 7146-7159. https://doi.org/10.1093/jxb/eraa419 (This article has been highlighted: Jameson, P.E. & Song., J. (2020) Will cytokinins underpin the second ‘Green Revolution’? J. Exp. Bot., 71, 6872–6875. https://doi.org/10.1093/jxb/eraa447)
Werner, S., Bartrina, I., Novák, O., Strnad, M., Werner, T. Schmülling, T. (2021) The cytokinin status of the epidermis regulates aspects of vegetative and reproductive development in Arabidopsis thaliana. Front. Plant Sci. 12, 613488. https://doi.org/10.3389/fpls.2021.613488
- The role of cytokinin in the shoot apical meristem
Cytokinin regulates size and activity of shoot apical meristems through the WUS/CLV pathway. However, the relevance of meristematic subdomains and the transcriptional regulation of target genes are still to be explored. Recently, we have started a project to identify the role of cytokinin signaling in different domains of the shoot apical meristem. This project is led by Bernadette Eichstädt.
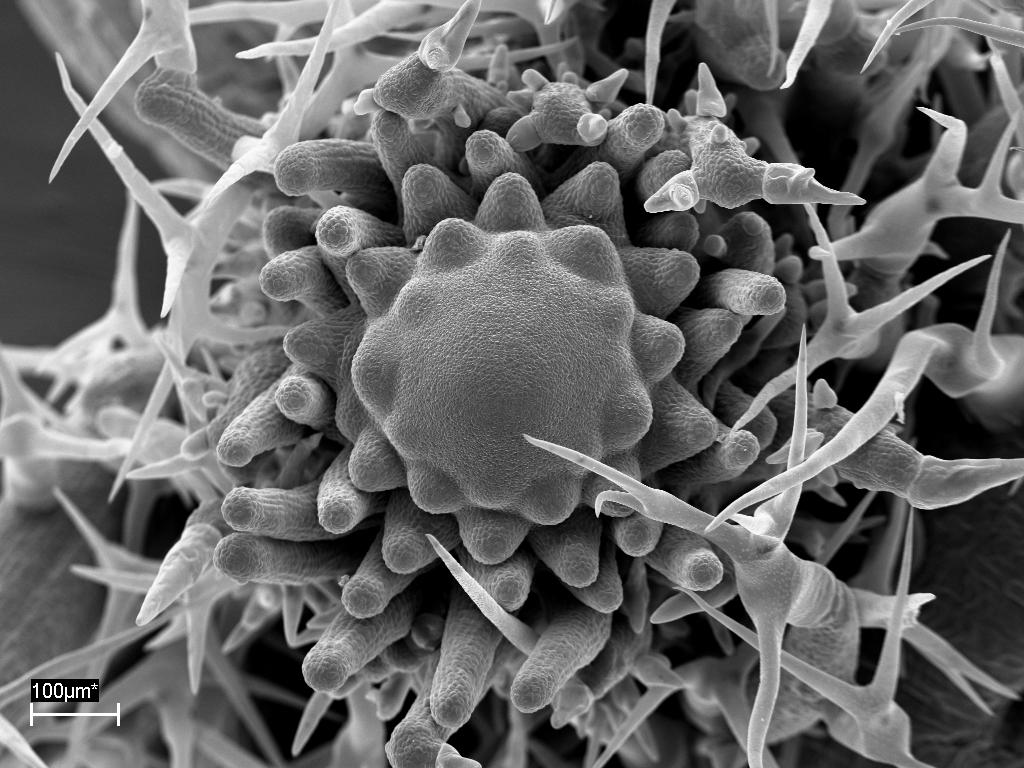
Enhanced cytokinin signaling increases
shoot apical meristem size of Arabidopsis (thesis E. Otto).
Rupp, H.M., Frank, M., Werner, T., Strnad, M., Schmülling, T. (1999) Increased steady state mRNA levels of the STM and KNAT1 homeobox genes in cytokinin overproducing Arabidopsis thaliana indicate a role for cytokinins in the shoot apical meristem. Plant J. 18, 357-363. https://doi:10.1046/j.1365-313x.1999.00472.x.
Bartrina, I., Otto, E., Strnad, M., Werner, T., Schmülling, T. (2011) Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23, 69-80. https://doi:10.1105/tpc.110.079079.
- Photoperiod stress, a novel abiotic stress in plants (project led by Anne Cortleven)
Several years ago, we have discovered that Arabidopsis plants respond to an extension of daylength by a strong stress response during the following night. Hallmarks of the photoperiod stress syndrome are an increased concentration of stress hormones and of reactive oxygen species (ROS). The stress response genes that are activated by photoperiod stress resemble those of the response to pathogens. Intriguingly, photoperiod stress treatment increases resistance to pathogens. It is thought that photoperiod stress is caused by malfunctioning of the circadian clock. Root-derived cytokinin alleviates and auxin increases photoperiod stress symptoms. This project is led independently by Anne Cortleven.
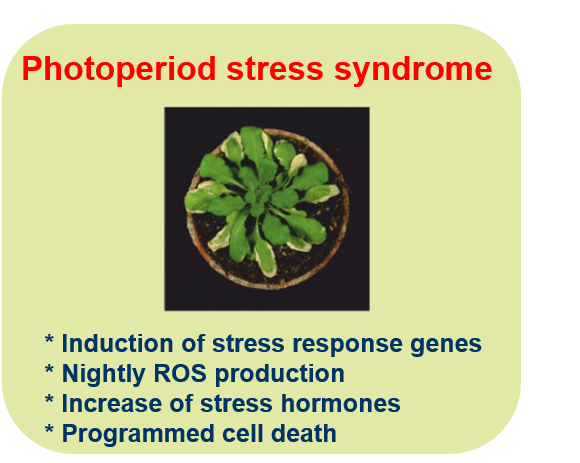
Hallmarks of the response to photoperiod stress.
Nitschke, S., Cortleven, A., Schmülling, T. (2017) Novel stress in plants by altering the photoperiod. Trends in Plant Sci. 22, 913-916. https://doi.org/10.1016/j.tplants.2017.09.005
Roeber, V.M., Bajaj, I., Rohde, M., Schmülling, T., Cortleven, A. (2021) Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 44, 645-664. https://doi.org/10.1111/pce.13948
Roeber, V.M., Schmülling, T., Cortleven, A. (2022) The photoperiod: handling and causing stress in plants. Frontiers in Plant Science 12, 781998. https://doi.org/10.3389/fpls.2021.781988
Nitschke, S., Cortleven, A., Iven, T., Feussner, I., Havaux, M., Riefler, M., Schmülling, T. (2016) Circadian stress regimes affect the circadian clock and cause jasmonic acid-dependent cell death in cytokinin-deficient Arabidopsis plants. Plant Cell 28, 1616-1639. https://doi:10.1105/tpc.16.00016.
Abuelsoud, W., Cortleven, A., Schmülling, T. (2020) Photoperiod stress induces an oxidative burst-like response and is associated with increased apoplastic peroxidase and decreased catalase activities. J. Plant Physiol., 153252. https://doi.org/10.1016/j.jplph.2020.153252
Frank, M., Cortleven, A., Novak, O., Schmülling, T. (2020) Root-derived trans-zeatin cytokinin protects Arabidopsis plants against photoperiod stress. Plant Cell Environm. 43, 2637–2649. https://doi.org/10.1111/pce.13860
Frank, M., Cortleven, A., Schmülling, T. (2022) The response to photoperiod stress in Arabidopsis thaliana depends on auxin acting as an antagonist to the protectant cytokinin. International Journal of Molecular Sciences 23, 2936. https://doi.org/10.3390/ijms23062936
Cortleven, A., Roeber, V.M., Frank, M., Bertels, J., Lortzing, V., Beemster, G., Schmülling, T. (2022) The transcriptomic landscape of the photoperiodic stress response in Arabidopsis thaliana resembles the response to pathogen infection. Frontiers in Plant Science 13, 838284. https://doi.org/10.3389/fpls.2022.838284
- Chemical priming of the plant stress response by cytokinin arabinosides
Cytokinin arabinosides (CK-A) are structurally similar to the plant hormone cytokinin but lack its growth regulatory activity. CK-As can induce pathogen-associated molecular patterns triggered immunity (PTI) in Arabidopsis thaliana (Bryksova et al., ACS Chem. Biol. 15, 1949–1963, 2020), indicating that CK-As might act as a chemical priming agent. Priming is the response of organisms to transient environmental (priming) stimuli in a way that prepares them for a better response to future stress. Priming may be caused by naturally occurring abiotic or biotic stresses; however, it can also be induced by chemicals. We are studying the ability of CK-As to prime Arabidopsis for defense against the hemibiotrophic pathogen Pseudomonas syringae pv. tomato DC3000 (Pst) and aim to elucidate the molecular mechanisms underlying priming by CK-As. This project is carried out by Martin Hönig (FEBS fellowship) in collaboration with the Department of Chemical Biology, Palacký University Olomouc.
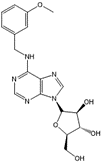
Chemical structure of a
cytokinin arabinoside (CK-A).
Hilker, M., Schwachtje, J., Baier, M., Balazadeh, S., Bäurle, I., Geiselhardt, S., Hincha, D.K., Kunze, R., Müller-Röber, B., Rillig, M.C., Rolff, J., Romeis, T., Schmülling, T., Steppuhn, A., van Dongen, J., Whitcomb, S.J., Wurst, S., Zuther, E., Kopka, J. (2016) Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. Camb. Philos. Soc. 91, 1118-1133. https://doi:10.1111/brv.12215
Hilker, M., Schmülling, T. (2019) Stress priming, memory and signalling in plants. Plant Cell Environm. 42, 753-761. https://doi.org/10.1111/pce.13526
Past projects
Activity and functions of cytokinin oxidases/dehydrogenases
Cytokinin signal transduction, functions and properties of cytokinin receptors
Regulation of root growth by cytokinin
Cytokinin as a regulator of chloroplast development
Response to cold stress
Tumor development in plants
Agrobacterium rhizogenes T-DNA genes
Funding of research
Our current research is funded by German research foundation (Deutsche Forschungsgemeinschaft, DFG) to explore photoperiod stress and by the Lieselotte and Prof. Dr. Kurt-Dietrich Krolow Foundation (Krolow Foundation) to study the role of CKX genes in the regulation of yield.
Past funding
A significant part of our funding has been received from DFG, both for numerous individual projects and the participation in a number of collaborative research centers (CRCs) and priority programs, includin
- CRC 973 Priming and memory of organismic responses to stress (2012 – 2022)
- Priority Program 1530: Flowering time control – from natural variation to crop improvement (2014-2018)
- CRC 429 Molecular physiology, energetics, and regulation of primary metabolism in plants (2002 - 2010)
- CRC 449 Structure and function of membrane integral receptors (2002 – 2010)
- Priority Program 1067 Molecular analysis of phytohormone activity (1999 – 2005)
- Priority Program 1005 Genetische und molekulare Aufklärung von Prozessen der Merkmalsausprägung bei Nutzpflanzen (1998 – 2001)
- CRC 446 Mechanism of cellular behaviour in eukaryotes (1997 – 2000)
- The Arabidopsis Functional Genomics Network (AFGN)
The laboratory has initiated and coordinated several international collaborative research projects, sponsored by the German Federal Ministry of Education and Research (BMBF) in the frame of the GABI, PLANT-KBBE, and PLANT2030 programs, including:
- GABI-GROWTH – Yield enhancement in oilseed rape (2008-2011)
- SEEDS – Yield enhancement in oilseed rape (2012 – 2016)
- ROOTS – Root enhancement for crop improvement (2010-2014)
We have participated in the BMBF-funded collaborative research project:
- PopMass – Development and use of novel gene technologies to increase biomass (2012-2015)
Further support has been obtained from Volkswagen Foundation and the Krolow Foundation for basic research, and from Syngenta, Bayer CropScience and BASF for applied projects.
The total sum of funding obtained is ~14 Mio Euro.


