Cytochrom P450 Enzymes: Metabolism & Application in Cancer Therapy
Understanding human metabolism is an essential aspect of modern drug design. Since research in this area typically focuses on 3A4, 2C9 and 2D6, many of the 57 functional human CYP enzymes are still largely uncharacterized. This previously neglected field offers many interesting possibilities for drug development (Drug Discov. Today, 26:2456-2464, 2021). In cooperation with Prof. Parr, we were able to make a substantial contribution to the understanding of CYP-mediated steroid metabolism, show new degradation pathways of olanzapine (3A43), and mechanistically explain various metabolites of alprazolam induced by polymorphisms (Front. Endocrinol., 12:633785, 2021, Xenobiotica, 52:413-425, 2022).
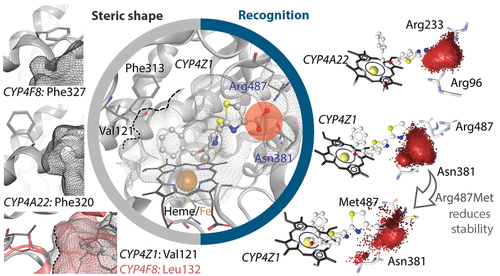
We were able to realize a first successful targeting of a hardly characterized cytochrome P450 enzyme to CYP4Z1: CYP4Z1 is overexpressed in breast cancer tissue and its immunoreactivity is associated with primary ovarian cancer. We were able to show for the first time that CYP4Z1 can catalyze the hydroxylation of lauric acid and myristic acid at four different positions. Irrespective of its natural function, the strong overexpression of CYP4Z1 in certain neoplasms makes it a possible target for a prodrug strategy to cure breast cancer and other CYP4Z1-positive tumors. Through extensive homology modeling in combination with molecular dynamics simulations, we were able to explain the catalysis of proluciferin substrates in the first step (Biochem. Pharmacol., 146:174-187, 2017). Our simulations could explain differences in activity upon mutation, led to the discovery of the key role of Arg487, and indicated the support of Asn381, which was confirmed in vitro. The data suggest that CYP4Z1 substrates are recruited by Arg487 and fixed by Asn381 and Arg487 (Biochem. Pharmacol., 174:113850, 2020). With this iteratively in silico and in vitro validated model, a new CYP4Z1 inhibitor with nanomolar activity could be developed ().
We would like to thank for financial support in this research area: