The impact of side-chain fluorination on proton-bound phenylalanine dimers: a cryogenic infrared spectroscopic study
Safferthal. M; Greis, K.; Chang, R.; Chang, C.W; Hoffmann, W.; Meijer, G.; von Helden, G.; Pagel, K.* – 2024
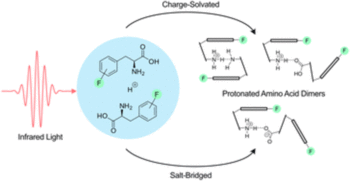
The incorporation of fluorine into amino acids is an important strategy to produce tailored building blocks with unique properties for peptide-based materials. Phenylalanine is frequently modified due to its role in cation–π interactions and the formation of amyloid fibres. Previous studies have utilized gas-phase vibrational spectroscopy to study interactions between canonical amino acids. In this study, we employ a combination of cryogenic gas-phase infrared spectroscopy and density functional theory to study the interactions in proton-bound dimers of side-chain fluorinated phenylalanines. Our findings reveal how the position and number of fluorine atoms affect the interactions and structures of the dimers. Monofluorinated phenylalanines adopt charge-solvated structures in which the two amino acids interact via their ammonium and amine functions (NH3+⋯NH2). The dimer with the perfluorinated side chain forms multiple charge-solvated and salt-bridged structures with varying interaction types. These structural changes are attributed to the significant reduction of electron density in the aromatic systems.
The incorporation of fluorine into amino acids is an important strategy to produce tailored building blocks with unique properties for peptide-based materials. Phenylalanine is frequently modified due to its role in cation–π interactions and the formation of amyloid fibres. Previous studies have utilized gas-phase vibrational spectroscopy to study interactions between canonical amino acids. In this study, we employ a combination of cryogenic gas-phase infrared spectroscopy and density functional theory to study the interactions in proton-bound dimers of side-chain fluorinated phenylalanines. Our findings reveal how the position and number of fluorine atoms affect the interactions and structures of the dimers. Monofluorinated phenylalanines adopt charge-solvated structures in which the two amino acids interact via their ammonium and amine functions (NH3+⋯NH2). The dimer with the perfluorinated side chain forms multiple charge-solvated and salt-bridged structures with varying interaction types. These structural changes are attributed to the significant reduction of electron density in the aromatic systems.