Mechanistic insight into benzylidene-directed glycosylation reactions using cryogenic infrared spectroscopy
Chang, C.W.; Greis, K.; Prabhu, G. R. D.; Wehner, D.; Kirschbaum, C.; Ober, K.; Torres-Boy, A. Y. ; Leichnitz, S.; Meijer, G.; v. Helden, G.; Seeberger, P. H.; K. Pagel* – 2024
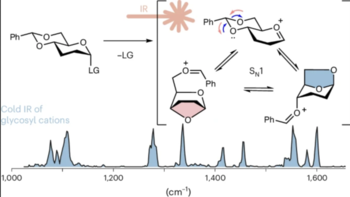
The stereoselective formation of 1,2-cis glycosidic linkages is challenging. The currently most widely used strategy for their installation uses 4,6-O-benzylidene protected building blocks. The stereoselectivity of this reaction is thought to be driven by a covalent intermediate, which reacts via an SN2 mechanism. However, the role of cationic SN1-type intermediates in this reaction is unclear. Here, we elucidate the structure of glycosyl cations carrying 4,6-O-benzylidene groups using cryogenic infrared ion spectroscopy and computational methods. The data reveal that the intermediates unexpectedly form anhydro cations, which correlates well with the stereoselective outcome of SN1-type glycosylations. The study highlights how cryogenic infrared spectroscopy can unravel novel intermediates in sugar chemistry and how this structural data can be linked to reactions in solution.
The stereoselective formation of 1,2-cis glycosidic linkages is challenging. The currently most widely used strategy for their installation uses 4,6-O-benzylidene protected building blocks. The stereoselectivity of this reaction is thought to be driven by a covalent intermediate, which reacts via an SN2 mechanism. However, the role of cationic SN1-type intermediates in this reaction is unclear. Here, we elucidate the structure of glycosyl cations carrying 4,6-O-benzylidene groups using cryogenic infrared ion spectroscopy and computational methods. The data reveal that the intermediates unexpectedly form anhydro cations, which correlates well with the stereoselective outcome of SN1-type glycosylations. The study highlights how cryogenic infrared spectroscopy can unravel novel intermediates in sugar chemistry and how this structural data can be linked to reactions in solution.