Studying the Intrinsic Reactivity of Chromanes by Gas-Phase Infrared Spectroscopy
Kirschbaum, C.; Greis, K.; Torres-Boy, A.Y.; Riedel, J.; Gewinner, S.; Schöllkopf, W.; Meijer, G.; von Helden, G.; Pagel, K.* – 2024
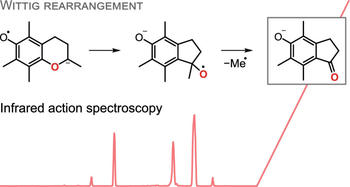
Tandem mass spectrometry is routinely used for the structural analysis of organic molecules, but many fragmentation reactions are not well understood. Because several potential structures can correspond to a measured mass, the assignment of product ions is ambiguous using mass spectrometry alone. Here, we combine mass spectrometry with high-resolution gas-phase infrared spectroscopy and computational chemistry tools to identify product ion structures and derive collision-induced fragmentation mechanisms of the chromane derivatives Trolox and Methyltrolox. We find that protonated Trolox and Methyltrolox fragment identically via dehydration and decarbonylation, while deprotonated ions display substantially diverging reactivities. For deprotonated Methyltrolox, we observe unusual radical fragmentation reactions and suggest a [1,2]-Wittig rearrangement involving aryl migration in the gas phase. Overall, the combined experimental and theoretical approach presented here revealed complex proton dynamics and intramolecular rearrangement reactions, which expand our understanding on structure–reactivity relationships of isolated molecules in different protonation states..
Tandem mass spectrometry is routinely used for the structural analysis of organic molecules, but many fragmentation reactions are not well understood. Because several potential structures can correspond to a measured mass, the assignment of product ions is ambiguous using mass spectrometry alone. Here, we combine mass spectrometry with high-resolution gas-phase infrared spectroscopy and computational chemistry tools to identify product ion structures and derive collision-induced fragmentation mechanisms of the chromane derivatives Trolox and Methyltrolox. We find that protonated Trolox and Methyltrolox fragment identically via dehydration and decarbonylation, while deprotonated ions display substantially diverging reactivities. For deprotonated Methyltrolox, we observe unusual radical fragmentation reactions and suggest a [1,2]-Wittig rearrangement involving aryl migration in the gas phase. Overall, the combined experimental and theoretical approach presented here revealed complex proton dynamics and intramolecular rearrangement reactions, which expand our understanding on structure–reactivity relationships of isolated molecules in different protonation states.