Direct Experimental Characterization of the Ferrier Glycosyl Cation in the Gas Phase
Greis, K.; Kirschbaum, C.; Leichnitz, S.; Gewinner, S.; Schöllkopf, W.; von Helden, G.; Meijer, G.; Seeberger, P. H.; Pagel, K* – 2020
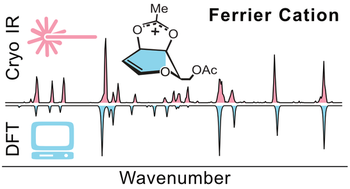
The Ferrier rearrangement reaction is crucial for the synthesis of pharmaceuticals. Although its mechanism was described more than 50 years ago, the structure of the intermediate remains elusive. Two structures have been proposed for this Ferrier glycosyl cation: a 1,2-unsaturated cation that is resonance-stabilized within the pyranose ring or a cation that is stabilized by the anchimeric assistance of a neighboring acetyl group. Using a combination of gas-phase cryogenic infrared spectroscopy in helium nanodroplets and first- principles density functional theory, we provide the first direct structural characterization of Ferrier cations. The data show that both acetylated glucal and galactal lead to glycosyl cations of the dioxolenium type.