Protomers of Benzocaine: Solvent and Permittivity Dependence
Warnke, S.; Seo, J.; Boschmans, J.; Sobott, F.; Scrivens, J. H.; Bleiholder, C.; Bowers, M. T.; Gewinner, S.; Schöllkopf, W.; Pagel, K.*; von Helden, G.* – 2015
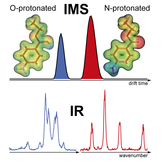
The immediate environment of a molecule can have a profound influence on its properties. Benzocaine, the ethyl ester of para-aminobenzoic acid, which finds an application as a local anesthetic (LA), is found to adopt in its protonated form at least two populations of distinct structures in the gas phase and their relative intensities strongly depend on the properties of the solvent used in the electrospray ionization (ESI) process. Here we combine IR-vibrational spectroscopy with ion mobility-mass spectrometry (IM-MS) to yield gas-phase IR spectra of simultaneously m/z and drift-time resolved species of benzocaine. The results allow for an unambiguous identification of two protomeric species - the N- and O-protonated form. Density functional theory (DFT) calculations link these structures to the most stable solution and gas-phase structures, respectively, with the electric properties of the surrounding medium being the main determinant for the preferred protonation site. The fact that the N-protonated form of benzocaine can be found in the gas phase is owed to kinetic trapping of the solution phase structure during transfer into the experimental setup. These observations confirm earlier studies on similar molecules where N- and O-protonation has been suggested.