Photodissociation of conformer-selected ubiquitin ions reveals site-specific cis/trans isomerization of proline peptide bonds
Warnke, S.; Baldauf, C.; Bowers, M.; Pagel, K.*; von Helden, G. – 2014
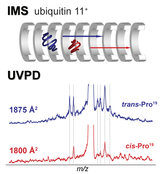
Ultraviolet photodissociation of gas-phase proteins (UVPD) has attracted increased attention in recent years. This growing interest is largely based on the fact that – in contrast to slow heating techniques such as collision induced dissociation (CID) - the cleavage propensity after absorption of UV light is distributed over the entire protein sequence, which can lead to a very high sequence coverage as required in typical top-down proteomics applications. However, in the gas phase proteins can adopt a multitude of distinct and sometimes coexisting conformations and it is not clear how this three-dimensional structure affects the UVPD fragmentation behavior. Using ion mobility – UVPD – mass spectrometry in conjunction with molecular dynamics simulations we provide the first experimental evidence that UVPD is sensitive to the higher order structure of gas-phase proteins. Distinct UVPD spectra were obtained for different extended conformations of 11+ ubiquitin ions. Assignment of the fragments showed, that the majority of differences arise from cis/trans isomerization of one particular proline peptide bond. Seen from a broader perspective these data highlight the potential of UVPD to be used for the structural analysis of proteins in the gas phase.
Spotlight in J. Am. Chem. Soc. 2014, 136 (29), 10173–10173 by Jeffrey M. Perkel, 'Probing Protein Structure with UVPD' DOI: 10.1021/ja507050z