Inhibition of peptide aggregation by means of enzymatic phosphorylation
Phosphorylation is one of the most important postranslational protein modifications in nature. Investigating the impact of the electronegative phosphate group on the conformation of peptides is of great interest for the elucidation of many pathologic events, such as tauopathies. Depending upon the location of this covalent modification, and the neighboring functional groups in the peptide, enzymatic phosphorylation can either stabilize or destabilize specific conformational states (Broncel, Chem. Eur. J., 2010).
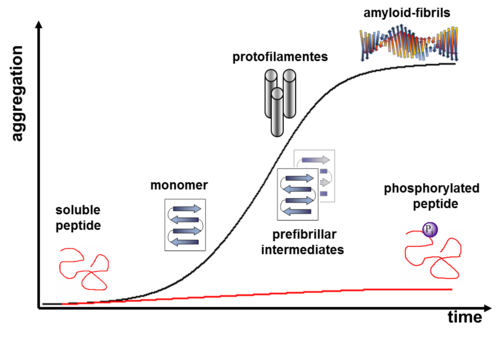
We have shown that incorporation of phosphoserines into a 26-residue, amyloid-forming model peptide precludes amyloid formation, and that enzymatic dephosphorylation of these peptides with phosphatase lambda (λ-APPase) can induce a conformational switch from a coiled coil structure to amyloids (Broncel, Chem. Eur. J., 2010; Broncel, Chem. Commun. 2010). Furthermore, we developed a peptide system that enables detailed amyloid-aggregation inhibition studies using cAMP dependent Proteinkinase A (PKA) (Figure 2). Further systematic studies of this kind will contribute significantly to our understanding of how phosphorylation influences pathways leading to peptide aggregation, and which structural interactions affect the conformation of a peptide during this process.