Infrared Spectroscopic and Theoretical Investigations of the OUF2 and OThF2 Molecules with Triple Oxo Bond Character
Yu Gong, Lester Andrews, Xuefeng Wang, Tobias Schlöder, Sebastian Riedel – 2012
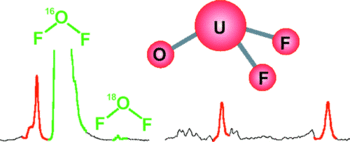
The terminal oxo species OUF₂ and OThF₂ have been prepared via the spontaneous and specific OF₂ molecule reactions with laser ablated uranium and thorium atoms in solid argon and neon. These isolated molecules are characterized by one terminal M-O and two F–M–F (M = U or Th) stretching vibrational modes observed in matrix isolation infrared spectra, which are further supported by density functional frequency calculations and CASPT2 energy and structure calculations. Both molecules have pyramidal structures with singlet (Th) and triplet (U) ground states. The molecular orbitals and metal–oxygen bond lengths for the OUF₂ and OThF₂ molecules indicate triple bond character for the terminal oxo groups, which are also substantiated by NBO analysis at the B3LYP level and by CASPT2 molecular orbital calculations. Dative bonding involving O₂p → Th₆d and Udf interactions is clearly involved in these oxoactinide difluoride molecules. Finally, the weak O–F bond in OF₂ as well as the strong U–O, U–F and Th–O, Th–F bonds make reaction to form the OUF₂ and OThF₂ molecules highly exothermic.