How Far Can We Go? Quantum-Chemical Investigations of Oxidation State +IX
Daniel Himmel, Carsten Knapp, Michael Patzschke, Sebastian Riedel – 2010
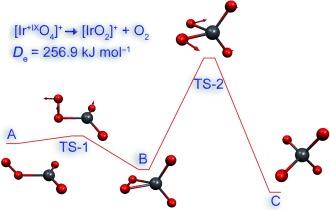
The highest known oxidation state of any element is +VIII. After the recent discovery of IrⱽᴵᴵᴵO₄ under cryogenic conditions, we have investigated the stability of cationic species [MO₄]⁺ (M=Rh, Ir, Mt). Such compounds would formally represent the new oxidation state +IX, which is experimentally unknown so far for the whole periodic table. [IrO₄]⁺ is predicted to be the most promising candidate. The calculated spin–orbit (SO) coupling shows only minor effects on the stability of the iridium species, whereas SO-coupling increases enormously for the corresponding Eka-Iridium (Meitnerium) complexes and destabilizes these.
Title
How Far Can We Go? Quantum-Chemical Investigations of Oxidation State +IX
Author
Daniel Himmel, Carsten Knapp, Michael Patzschke, Sebastian Riedel
Date
2010
Source(s)
Appeared in
Chem. Phys. Chem. 2010, 11, 4, 865 - 869
Citation
DOI: 10.1002/cphc.200900910
Language
eng