Behavior of BrO3F and ClO3F Toward Strong Lewis Acids and the Characterization of [XO2][SbF6] (X = Cl, Br) by Single Crystal X-ray Diffraction, Raman Spectroscopy, and Computational Methods
John F. Lehmann, Sebastian Riedel, Gary J. Schrobilgen – 2008
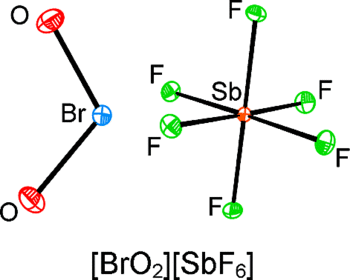
The interactions of BrO₃F and ClO₃F with the strong Lewis acids AsF₅ and SbF₅ were investigated. Although ClO₃F is unreactive toward AsF₅ and SbF₅, BrO₃F undergoes fluoride ion abstraction and O₂ elimination, accompanied by central halogen redn., to form [BrO₂][SbnF₅ₙ₊₁] (n≥1), rather than simple fluoride ion abstraction to form BrO₃⁺ salts. The geometric parameters of the BrO₂⁺ cation have been obtained in the solid state for the first time by a single-crystal X-ray diffraction study of [BrO₂][SbF₆] at -173 °C and are compared with those of ClO₂⁺ salts. Quantum-chem. calcns. have been used to arrive at the geometries and vibrational frequencies of XO₂⁺ and XO₃⁺ (X = Cl, Br) and have been compared with the exptl. values for XO₂⁺. The calcns. have also been used to account for the contrasting behaviors of ClO₃F and BrO₃F toward central halogen redn. in the presence of liq. SbF₅. The thermochem. stabilities of ClO₃⁺ and BrO₃⁺ salts of the AsF₆⁻, SbF₆⁻, Sb₂F₁₁⁻, and Sb3F₁₆⁻ were also investigated, which provided the fluoride ion affinities of AsF5, SbF5, Sb₂F₁₀, and Sb₃F₁₅ up to and including the CCSD(T) level of theory. These values are compared with the current literature values. Thermochem. studies indicate that XO₃⁺ formation by fluoride ion abstraction from XO₃F is not spontaneous under std. conditions whereas a concerted fluoride abstraction and O₂ elimination to give the XO₂⁺ cations is spontaneous to near thermally neutral. Failure to observe reactivity between ClO₃F and any of the aforementioned Lewis acid fluoride ion acceptors is attributed to a significant kinetic barrier to fluoride ion abstraction.