Muscle development in Neurofibromatosis type I
Neurofibromatosis type I (NF1) is a multi-system disease caused by mutations in the NF1 gene. NF1 encodes a RAS-GAP protein, Neurofibromin, which negatively regulates RAS signaling. Besides neuroectodermal malformations and tumours, the skeletal system is often affected in NF1 patients, scoliosis and long bone dysplasias being a main cause of considerable morbidity. Interestingly, a reduction of muscle strength and size has been reported in NF1 patients. However it remained unclear whether the observed muscle weakness was a consequence of the skeletal ramifications developing during puberty and early adulthood or if there was a muscular phenotype before the onset of a bone phenotype.
We recently demonstrated a primary defect of embryonic muscle development in a mouse model for NF1 (Kossler et al. 2011).
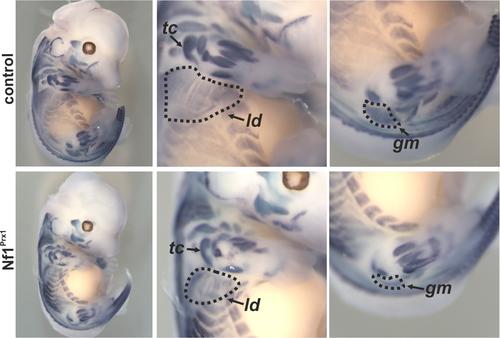
In-situ hybridisation for MyoD at embryonic day 13.5 demonstrating muscle patterning and size defects in the Nf1Prx1 mouse mutant. The magnifications show fore- and hindlimbs with reduction of muscle area visible e.g. for the latissimus dorsi muscle (ld), the triceps muscle (tc) or the gluteus maximus muscle (gm). Modified from Kossler et al. 2011.
This defect resulted in a continuous myopathy in Nf1Prx1 mice displaying dystrophic features such as muscle mass reduction, muscle strength reduction and connective tissue overgrowth.
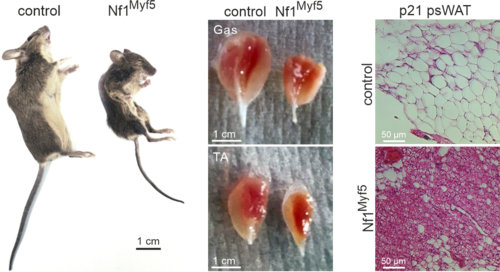
Following this, we conditionally inactivated Nf1 specifically in muscle via Myf5-Cre (Nf1Myf5). This led to a postnatal muscle growth impairment with hallmarks of altered muscle proteostasis leading to smaller, atrophic muscles. Furthermore, loss of NF1 caused a metabolic shift in muscle fibers from a glycolytic to a more oxidative type. This was concomitant to increased consumption and mobilization of fatty acids, leading to an overall cachectic phenotype (Wei et al. 2020).
Appearance of muscle-specific Nf1 KO mice, size of muscles (Gas: Gastrocnemius; TA: Tibialis anterior) and histological appearance of white adipose tissue (psWAT: posterior subcutaneous white adipose tissue). Adapted from (Wei et al. 2020).
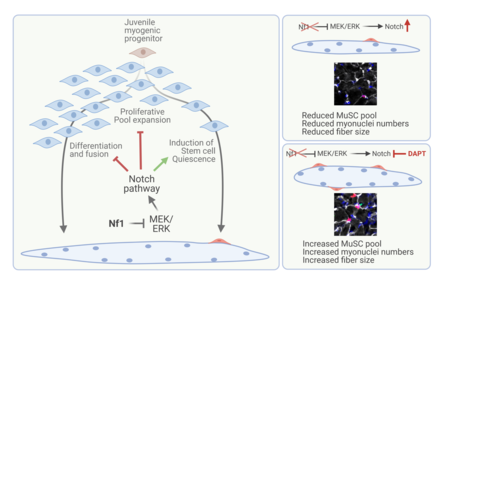
Very recently, we analysed myogenic progenitor behaviour in Nf1Myf5 mice, and surprisingly found, that the myofiber phenotype described above could be fully traced back to aberrant programming of muscle progenitor cells during the first weeks of postnatal life. Loss of Nf1 led, via exacerbated Notch signalling, to a precocious entry into muscle stem cell quiescence concomitant to metabolic reprogramming resulting in glycolytic shutdown, which was transmitted to muscle fibers. (Wei et al. 2024).
Schematic summarizing the role of NF1/MEK/ERK signalling and its interplay with Notch signalling in early postnatal mouse myogenic progenitors. Loss of NF1 results in exacerbated Notch signalling driving stem cell quiescence at the expense of pool expansion and myofiber formation. The defect can in part be rescued by early postnatal Notch pathway inhibition via DAPT. Adapted from (Wei et al. 2024).