Research
Bottanelli group – Membrane trafficking
The overarching goal of the lab is to understand the mechanisms of membrane homeostasis in health and disease. The development and differentiation of cells and tissues relies on the correct delivery of receptors, transporters, ion channels and important signalling molecules to the plasma membrane (PM) via the secretory pathway. Although individual components of the sorting machinery are identified, the dynamics and mechanisms of material exchange between the Golgi apparatus and neighboring organelles are still elusive. To be able to dissect molecular processes in the crowded cellular cytoplasm in living cells, we developed labelling methods for emission depletion (STED) super-resolution imaging and a pipeline for the rapid generation of CRISPR-Cas9 knock ins (Bottanelli et al., 2016, 2017). These methods unlocked the possibility to image dynamics at sub-50 nm spatial resolution and under near-physiological cellular conditions.
The lab has a long-standing interest in understanding the function of ARF GTPases, major regulators of intracellular membrane homeostasis (Wong-Dilworth et al. 2023). Dynamic nanoscale imaging of endogenously tagged ARFs and trafficking machinery is revealing unexpected and unexplored sorting mechanisms. In Wong-Dilworth et al. 2023 (BioRxiv, under review for EMBO journal) we dissect the mechanisms of ER-to-Golgi transport in human cells by following the dynamics of endogenously tagged machinery components using live-cell STED super-resolution microscopy combined with functional trafficking assays. Our results suggest that cargo percolates through a dynamic network of ARF4 tubules and Golgi-tethered ER-Golgi intermediate compartments (ERGICs) are the entry gateway into the Golgi apparatus. In Stockhammer, Adarska et al., 2023 (BioRxiv, under revision for Nature Cell Biology), we functionally characterize a novel sorting compartment that coordinates clathrin-dependent post-Golgi trafficking which we named ARF1 compartment.
An HFSP early career grant allowed us to initiate a new area of research focused on understanding how signalling molecules dynamically re-organize on membranes during immune signalling with unprecedented detail. Our findings fill important gaps in our understanding of fundamental membrane processes. Moving forward, we aim to explore how extracellular cues, such as nutrient availability, polarization and immune signalling re-wire membrane flow to sustain membrane homeostasis.
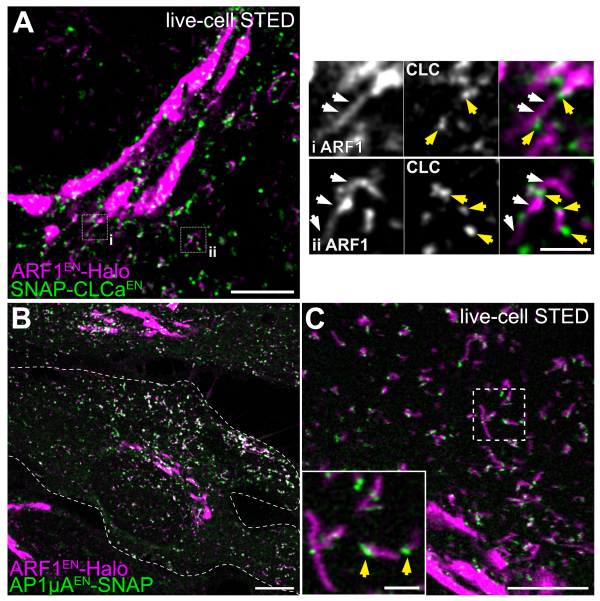
ARF1 orchestrates endocytic and exocytic traffic via multi-functional tubular endosomal compartments. Gene edited ARF1 and CLCa highlight sorting tubular compartments (white arrows) decorated by clathrin clusters (yellow arrows, A). AP-1 is associated to ARF1 compartments (B) and nano-domains of AP-1 (yellow arrows) are seen on the tubules with live-cell STED. Scale bars are 5 µm in A and 1 µm in the crop, 10 µm in B. Adapted from Wong-Dilworth et al. 2023 and Stockhammer, Adarska et al. 2023.
