Synthesis, structure and thermolysis of oxazagermines and oxazasilines
F. Dannenberg, G. Thiele, E. Dornsiepen, S. Dehnen, M. Mehring – 2017
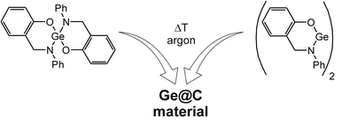
Seven new spirocyclic amido alkoxides of silicon and germanium were synthesized by the reactions of substituted 2-(aminomethyl)phenols with silicon(IV) and germanium(IV) chloride, respectively. The spirocyclic compounds 1–7 exhibit the general structural motif M[OC6H3(CH2NR1)-2-R2-4]2 [M = Si, R1 = Ph, R2 = H (1), Me (2), Br (3); M = Ge, R1 = Ph, R2 = H (4), Me (5), Br (6); M = Ge, R1 = i-Pr, R2 = H (7)]. The use of 2-(ethylamino)benzylalcohol and germanium(IV) chloride as starting materials afforded the spirocyclic compound Ge[EtNC6H4(CH2O)-2]2 (8). The structurally related germylene, germanium(II)-2-(phenylamidomethyl)phenolate (9), was prepared by the reaction of Ge[N(SiMe3)2]2 with 2-(phenylaminomethyl)phenol. The tetravalent compounds were isolated as racemates, with the exception of the spirocyclic germanium compound 8, for which the R and the S isomers crystallized separately. The compounds were characterized by single-crystal X-ray diffraction analysis, NMR spectroscopy (1H, 13C{1H}, 29Si{1H} for 1–3), IR spectroscopy, and TGA analysis. Porous Ge@C composites were observed after the carbonization of compounds 4–9 under argon. The as-prepared Ge@C materials were analyzed by elemental analysis, nitrogen physisorption measurements, powder X-ray diffraction analysis, Raman spectroscopy, and SEM/EDX analysis.