About the Syntheses of Chalcogenidometalates by in‐situ Reduction with Elemental Alkali Metals
G. Thiele, L. Vondung, S. Dehnen – 2015
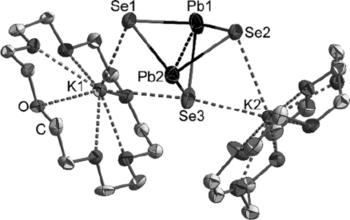
Reduction of chalcogen‐rich Pb:Ch (1:2) phases in ethane‐1,2‐diamine (en) by elemental alkali metals results in the formation of solutions of [Pb2Ch3]2– of high purity and abundance. In contrast, application of the same reaction conditions to a binary Bi:Te (1:2) phase yields the mononuclear [BiTe3]3– anion. Instead of the expected [Tl2Te3]4– or [Tl2Te2]2– anions, analogous reactions with a Tl:Te (1:1) phase end up with a C–C‐bond cleavage of the solvent en with formation of a salt of the telluridocyanate (N≡C–Te)– anion. Side reactions of en and elemental cesium are presented and metalate solutions are investigated with NMR spectroscopy.
Title
About the Syntheses of Chalcogenidometalates by in‐situ Reduction with Elemental Alkali Metals
Author
G. Thiele, L. Vondung, S. Dehnen
Date
2015
Citation
Z. Anorg. Allg. Chem. 2015, 641, 247.