Unravelling the Role of the Pentafluoroorthotellurate Group as a Ligand in Nickel Chemistry
A. Pérez-Bitrián, K. F. Hoffmann, K. B. Krause, G. Thiele, C. Limberg, S. Hasenstab-Riedel – 2022
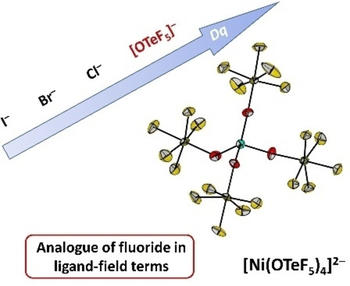
The pentafluoroorthotellurate group (teflate, OTeF5) is able to form species, for which only the fluoride analogues are known. Despite nickel fluorides being widely investigated, nickel teflates have remained elusive for decades. By reaction of [NiCl4]2– and neat ClOTeF5, we have synthesized the homoleptic [Ni(OTeF5)4]2– anion, which presents a distorted tetrahedral structure, unlike the polymeric [NiF4]2–. This high-spin complex has allowed the study of the electronic properties of the teflate group, which can be classified as a weak/medium-field ligand, and therefore behaves as the fluoride analogue also in ligand-field terms. The teflate ligands in [NEt4]2[Ni(OTeF5)4] are easily substituted, as shown by the formation of [Ni(NCMe)6][OTeF5]2 by dissolving it in acetonitrile. Nevertheless, careful reactions with other conventional ligands have enabled the crystallization of nickel teflate complexes with different coordination geometries, i.e. [NEt4]2[trans-Ni(OEt2)2(OTeF5)4] or [NEt4][Ni(bpyMe2)(OTeF5)3].