Team Rost
Our research concerns the synthesis and characterisation of low-coordinate alkali metal chalcogenido metalates Ax[MyChz] (A = Li-K, M = Fe, Hg, Ch = O, S). Alkali metal oxidoferrates reported in literature display structures including isolated or tetrahedrally connected coordination motifs of the iron(III) cations by the oxygen anions (Figure 1).[1,2] In contrast, alkali metal chalcogenido mercurates exhibit a dumb-bell coordination of the mercury(II) cation by the oxygen anions (Figure 1).[3]
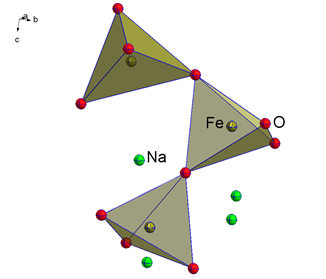
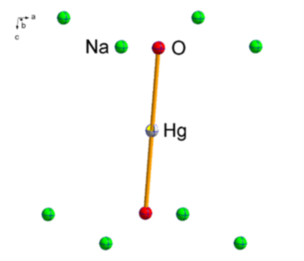
Figure 1: Excerpt from the crystal structure of Na4[Fe2O5] with connected [FeO4]−-tetrahedra (left) and an excerpt from the crystal structure of Na2[HgO2]with dumb-bell coordinated mercury(II) cations (right).[3,4] Displacement ellipsoids are shown at the 70% probability level.
Our goals include the isolation of low-coordinate alkali metal oxido ferrates with iron(I) and iron(II) cations in linear and trigonal planar coordination, respectively. As of yet, examples of one of each have been reported in literature.[5,6] Commonly, either the pure metals or metal oxides are used as starting materials (Scheme 1).
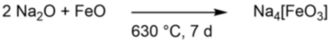
Scheme 1: Reaction of sodium oxide and iron(II) oxide in a high-temperature furnace
Our main working technique includes solid-state syntheses at high temperatures. When working with air-stable compounds the reactions are carried out in crucibles made from corundum in high-temperature furnaces. For reactions in furnaces under the exclusion of air and moisture, we apply quartz tubes, if required with steel autoclaves. The duration of solid-state reactions carried out in furnaces can vary from a few hours to several weeks. For quicker conversions with reaction times of 3-20 minutes, we apply a hand torch running on a mixture of methane and oxygen to heat up compounds in quartz ampoules up to 1400 °C.
In solvothermal syntheses with temperatures of 120-180 °C and under slightly increased pressure, the reaction products are either recrystallised or reacted with e.g. ammonium salts. The goal of this metathesis reaction is an exchange of the alkali cation with a sterically more demanding, asymmetric, organic cation, with the aim of increasing the chalcogenido metalate’s solubility in common organic solvents (Scheme 2). In addition, we are investigating the solubility of the compounds in deep eutectic solvents.

Scheme 2: Metathesis reaction of sodium mercurate(II) and tetraethylammonium chloride.
Reaction products are mostly characterised via single crystal or powder X-ray diffraction analysis. These analytical techniques enables the determination of structures of novel compounds (single crystal X-ray diffraction analysis), as well as the evaluation of the compounds’ purity (powder X-ray diffraction analysis).
Additional characterisation techniques include investigations of the opto-electronic properties of the compounds, e.g. via UV/Vis-, IR- or Raman-spectroscopy.
[1] G. Brachtel, R. Hoppe, Z. Anorg. Allg. Chem. 1978, 446, 87.
[2] G. Brachtel, R. Hoppe, Z. Anorg. Allg. Chem. 1978, 446, 77.
[3] G. Brachtel, R. Hoppe, Naturwissenschaften 1977, 64, 271.
[4] R. Hoppe, H.-J. Röhrborn, Z. Anorg. Allg. Chem. 1964, 329, 110.
[5] F. Bernhardt, R. Hoppe, Z. Anorg. Allg. Chem. 1993, 619, 969.
[6] R. Hoppe, H. Rieck, Z. Anorg. Allg. Chem. 1977, 437, 95.
Keywords
- Alkalimetall
- Bismut
- Eisen
- Nickel
- niedervalente Chalkogenidometallate
- Reduktion
- Schwefel
