Team Elektride
The electride project investigates the solvation and stabilisation of electrons in primary amines. Alkali metals, such as sodium and potassium, are oxidised, while the corresponding electrons are stabilised by the solvent, e.g. ammonia, yielding a characteristic blue colour (Scheme 1).[1,2].
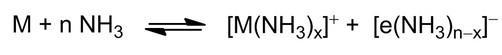
Scheme 1: Solvation of alkali metal cations and electrons in liquid ammonia.
Naked electrons are one of the strongest reducing agents, which results in unprecedented reactivities. Electrides can be applied in the synthesis of novel metalides (metals in negative oxidation states) or for the attempted reduction of compounds, that are in fact known to be unreactive.
[Cs(18C6)2]e− (18C6 = 18-Crown-6) was the first crystalline electride which was isolated, further electrides with sequestring agents have been characterised since.[3,4] Through similar approaches alkalides have been synthesised and crystallised, such as the first alkalide [Na(C222)]Na (C222 = [2.2.2]cryptand).[5,6]
One of our goals is the synthesis and crystallisation of metal-free electrides. We have been examining tetraalkylammonium salts for potential cations for this metathesis reaction (Scheme 2). Besides the dissolution of alkali metals, another approach to electride solutions lies in electrolysis in primary amines.
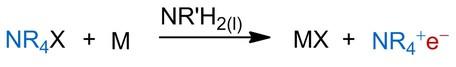
Schema 2: Metathesis reaction of tetraalkylammonium salts and alkali metals for the formation of electrides.
Figure 1 shows a reaction mixture with the characteristic blue colouring and metallic shine of an electride solution, as well as the formation of dendrites along the glass walls of the vessel.

Figure 1: Reaction mixture with electrides and the formation of dendrites along the glass walls of the vessel.
There are many experimental parameters, which can be tuned and the challenge lies in determining the ideal conditions for stabilising the electrons. The following techniques are included: drying solvents and salts, working at low temperatures of −30 - −90 °C, working with liquefied gases and working under the strict exclusion of air and moisture.
Reaction products are mostly characterised via powder or single crystal X-ray diffraction analysis, though other common analytical methods such as NMR-, electron EPR-spectroscopy or elemental analysis can also be pursued.
[1] J. L. Dye, Phil. Trans. R. Soc. A 373: 20140174.
[2] J. L. Dye, Science 2003, 301, 607.
[3] J. M. Ceraso, M. Tak Lok, B. L. Barnett, F. J. Tehan, J. L. Dye, J. Am. Chem. Soc.1974, 96, 608.
[4] B. Van Eck, L. Dinh Le, D. Issa, J. L. Dye, Inorg. Chem. 1982, 21, 1966.
[5] F. J. Tehan, B. L. Barnett, J. L. Dye, J. Am. Chem. Soc. 1974, 96, 7203.
[6] S. B. Dawes, D. L. Ward, R. He Huang, J. L. Dye, J. Am. Chem. Soc. 1986, 108, 3534.
Keywords
- Elektride
- Reduktion
