Neuronal Control of Metabolism through Nutrient-Dependent Modulation of Tracheal Branching
Gerit A. Linneweber, Jake Jacobson, Karl Emanuel Busch, Bruno Hudry, Christo P. Christov, Dirk Dormann, Michaela Yuan, Tomoki Otani, Elisabeth Knust, Mario de Bono, Irene Miguel-Aliaga – 2014
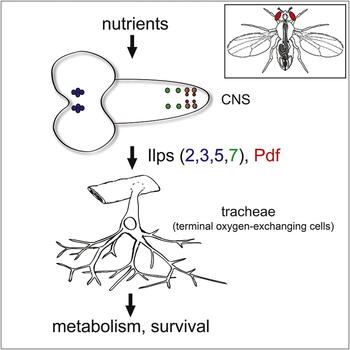
During adaptive angiogenesis, a key process in the etiology and treatment of cancer and obesity, the vasculature changes to meet the metabolic needs of its target tissues. Although the cues governing vascular remodeling are not fully understood, target-derived signals are generally believed to underlie this process. Here, we identify an alternative mechanism by characterizing the previously unrecognized nutrient-dependent plasticity of the Drosophila tracheal system: a network of oxygen-delivering tubules developmentally akin to mammalian blood vessels. We find that this plasticity, particularly prominent in the intestine, drives—rather than responds to—metabolic change. Mechanistically, it is regulated by distinct populations of nutrient- and oxygen-responsive neurons that, through delivery of both local and systemic insulin- and VIP-like neuropeptides, sculpt the growth of specific tracheal subsets. Thus, we describe a novel mechanism by which nutritional cues modulate neuronal activity to give rise to organ-specific, long-lasting changes in vascular architecture.
title = {Neuronal Control of Metabolism through Nutrient-Dependent Modulation of Tracheal Branching},
journal = {Cell},
volume = {156},
number = {1},
pages = {69-83},
year = {2014},
issn = {0092-8674},
doi = {https://doi.org/10.1016/j.cell.2013.12.008},
url = {https://www.sciencedirect.com/science/article/pii/S0092867413015407},
author = {Gerit A. Linneweber and Jake Jacobson and Karl Emanuel Busch and Bruno Hudry and Christo P. Christov and Dirk Dormann and Michaela Yuan and Tomoki Otani and Elisabeth Knust and Mario de Bono and Irene Miguel-Aliaga},
abstract = {Summary
During adaptive angiogenesis, a key process in the etiology and treatment of cancer and obesity, the vasculature changes to meet the metabolic needs of its target tissues. Although the cues governing vascular remodeling are not fully understood, target-derived signals are generally believed to underlie this process. Here, we identify an alternative mechanism by characterizing the previously unrecognized nutrient-dependent plasticity of the Drosophila tracheal system: a network of oxygen-delivering tubules developmentally akin to mammalian blood vessels. We find that this plasticity, particularly prominent in the intestine, drives—rather than responds to—metabolic change. Mechanistically, it is regulated by distinct populations of nutrient- and oxygen-responsive neurons that, through delivery of both local and systemic insulin- and VIP-like neuropeptides, sculpt the growth of specific tracheal subsets. Thus, we describe a novel mechanism by which nutritional cues modulate neuronal activity to give rise to organ-specific, long-lasting changes in vascular architecture.}
}