The Crystal Structure of α-F2: Solving a 50 Year Old Puzzle Computationally
Carsten Müller, Stefan Mattsson, Beate Paulus, Frenio A. Redeker, Helmut Beckers, Sebastian Riedel – 2019
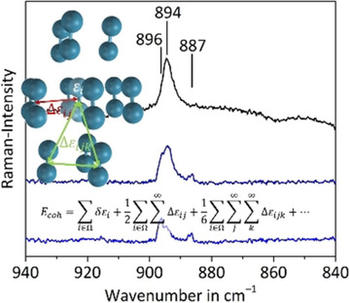
The cohesive energy of α‐fluorine, with C2/c space group symmetry, was calculated at benchmark quality by applying the method of increments. The known experimental X‐ray structure data needed to be refined, since the reported intramolecular bond length was unrealistically large. At the CCSD(T) level, including corrections for zero‐point energy, the basis set superposition error, and extrapolated to the complete basis set limit, a cohesive energy of −8.72 kJ mol⁻¹ was calculated, which agrees well with the 0 K‐extrapolated experimental value of −8.35 kJ mol⁻¹. Comparison of the C2/c structure with a Cmca structure, isotypic to that of chlorine, bromine, and iodine reveals that the origin of the different structure of solid fluorine, compared to the heavier halogens, is the lack of significantly stabilizing σ‐hole interactions. In addition, the wave numbers of the stretching mode in solid fluorine were calculated at coupled cluster level and compared to newly recorded Raman spectra of condensed fluorine. Both experiment and calculation indicate a slight up‐shift for the stretching mode by 2 or 5 cm⁻¹, respectively, with respect to a free F₂ molecule in the gas phase.