Polybromide Dianions and Networks Stabilized by Fluorinated Bromo(triaryl)phosphonium Cations
Lisa Mann, Gene Senges, Karsten Sonnenberg, Heike Haller, Sebastian Riedel – 2018
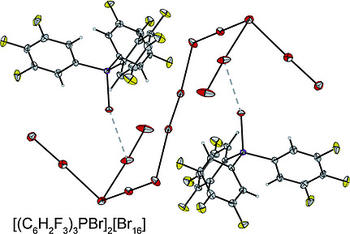
Triarylphosphanes react with elemental bromine to give triarylbromophosphonium bromides. With an excess of bromine larger polybromides are formed. The influence of the stepwise fluorination of the cation on the formed polybromide anion was investigated. The polybromide dianions and networks [(C₆H₅)₃PBr]₂[Br₁₄], [(C₆H₄F)₃PBr]₂[Br₁₄], [(C₆H₄F)₃PBr][Br₁₁], [(C₆H₂F₃)₃PBr]₂[Br₁₆], and [(C₆F₅)₃PBr]₂[Br₂₀]·Br₂ were obtained and characterized by Raman spectroscopy, single‐crystal X‐ray structure determination and quantum‐chemical calculations at the RI‐B3LYP‐D3/def2‐TZVPP level. Additionally, two further [Br₂₀]²⁻ dianions, [BMDIM][Br₂₀] {BMDIM = 1,12‐bis[(1‐methylbenzimidazolium)‐3‐yl]dodecane} and [AsPh₄]₂ [Br₂₀]·0.5Br₂, are presented and compared to all known eicosabromide dianions.