Isolation and Structural Characterization of Eightfold Protonated Octacyanometalates [M(CNH)8]4+ (M=MoIV, WIV) from Superacids
Malte Sellin, Valérie Marvaud, Moritz Malischewski – 2020
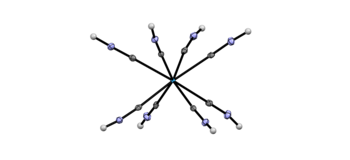
Octacyanometalates K4[Mo(CN)8] and K4[W(CN)8] are completely protonated in superacidic mixtures of anhydrous hydrogen fluoride and antimony pentafluoride. The resulting hydrogen isocyanide complexes [Mo(CNH)8]4+ [SbF6]−4 and [W(CNH)8]4+ [SbF6]−4 are the first examples of eight‐coordinate homoleptic metal complexes containing hydrogen isocyanide (CNH) ligands. The complexes were crystallographically characterized, revealing hydrogen‐bonded networks with short N⋅⋅⋅H⋅⋅⋅F contacts. Low‐temperature NMR measurements in HF confirmed rapid proton exchange even at −40 °C. Upon protonation, ν(C≡N) increases of about 50 cm−1 which is in agreement with DFT calculations.
Octacyanometalates K4[Mo(CN)8] and K4[W(CN)8] are completely protonated in superacidic mixtures of anhydrous hydrogen fluoride and antimony pentafluoride. The resulting hydrogen isocyanide complexes [Mo(CNH)8]4+ [SbF6]−4 and [W(CNH)8]4+ [SbF6]−4 are the first examples of eight‐coordinate homoleptic metal complexes containing hydrogen isocyanide (CNH) ligands. The complexes were crystallographically characterized, revealing hydrogen‐bonded networks with short N⋅⋅⋅H⋅⋅⋅F contacts. Low‐temperature NMR measurements in HF confirmed rapid proton exchange even at −40 °C. Upon protonation, ν(C≡N) increases of about 50 cm−1 which is in agreement with DFT calculations.