An Infrared Spectroscopy Approach to Follow β-Sheet Formation in Peptide Amyloid Assemblies
Seo, J.; Hoffmann, W.; Warnke, S.; Huang, X.; Gewinner, S.; Schöllkopf, W.; Bowers, M. T.; von Helden, G.;* and Pagel, K.* – 2017
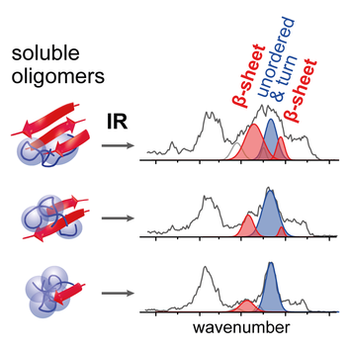
Amyloidogenic peptides and proteins play a crucial role in a variety of neurodegenerative disorders such as Alzheimer's and Parkinson's disease. These proteins undergo a spontaneous transition from a soluble, often partially folded form, into insoluble amyloid fibrils that are rich in β-sheets. Increasing evidence suggests that highly dynamic, polydisperse folding intermediates, which occur during fibril formation, are the toxic species in the amyloid-related diseases. Traditional condensed-phase methods are of limited use for characterizing these states because they typically only provide ensemble averages rather than information about individual oligomers. Here we report the first direct secondary-structure analysis of individual amyloid intermediates using a combination of ion mobility spectrometry–mass spectrometry and gas-phase infrared spectroscopy. Our data reveal that oligomers of the fibril-forming peptide segments VEALYL and YVEALL, which consist of 4–9 peptide strands, can contain a significant amount of β-sheet. In addition, our data show that the more-extended variants of each oligomer generally exhibit increased β-sheet content.
The paper was featured in the following articles:
Tagesspiegel report: http://www.tagesspiegel.de/themen/freie-universitaet-berlin/nachrichten-kurz-notiert-neues-aus-der-freien-universitaet/12690134.html