Elucidating reactive sugar-intermediates by mass spectrometry
Chang, C.W; Wehner, D.; Prabhu, G.R.D; Moon, E.; Safferthal, M.; Bechtella, L.; Österlund, N.; Vos, G.; Pagel, K.* – 2025
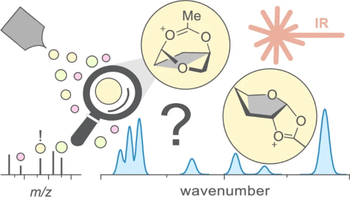
The stereoselective introduction of glycosidic bonds is one of the greatest challenges in carbohydrate chemistry. A key aspect of controlling glycan synthesis is the glycosylation reaction in which the glycosidic linkages are formed. The outcome is governed by a reactive sugar intermediate - the glycosyl cation. Glycosyl cations are highly unstable and short-lived, making them difficult to study using established analytical tools. However, mass-spectrometry-based techniques are perfectly suited to unravel the structure of glycosyl cations in the gas phase. The main approach involves isolating the reactive intermediate, free from external influences such as solvents and promoters. Isolation of the cations allows examining their structure by integrating orthogonal spectrometric and spectroscopic technologies. In this perspective, recent achievements in gas-phase research on glycosyl cations are highlighted. It provides an overview of the spectroscopic techniques used to probe the glycosyl cations and methods for interpreting their spectra. The connections between gas-phase data and mechanisms in solution synthesis are explored, given that glycosylation reactions are typically performed in solution.
The stereoselective introduction of glycosidic bonds is one of the greatest challenges in carbohydrate chemistry. A key aspect of controlling glycan synthesis is the glycosylation reaction in which the glycosidic linkages are formed. The outcome is governed by a reactive sugar intermediate - the glycosyl cation. Glycosyl cations are highly unstable and short-lived, making them difficult to study using established analytical tools. However, mass-spectrometry-based techniques are perfectly suited to unravel the structure of glycosyl cations in the gas phase. The main approach involves isolating the reactive intermediate, free from external influences such as solvents and promoters. Isolation of the cations allows examining their structure by integrating orthogonal spectrometric and spectroscopic technologies. In this perspective, recent achievements in gas-phase research on glycosyl cations are highlighted. It provides an overview of the spectroscopic techniques used to probe the glycosyl cations and methods for interpreting their spectra. The connections between gas-phase data and mechanisms in solution synthesis are explored, given that glycosylation reactions are typically performed in solution.