Molecular Diversity - Emergent Properties in Chemical Reactivity Networks
Course No. 21 234
As the course aims at a highly interactive format, it will - if Corona rules allow - be taught in presence. Please send - besides your registration with Campus Management - an email notice to c.schalley@fu-berlin.de to indicate your participation.
Introduction: Systems Chemistry & Molecular Diversity
In 2005, the term "Systems Chemistry" appeared in a conference on Prebiotic Chemistry and Early Evolution. This new field of chemical research has its roots in a number of different areas such as dynamic combinatorial chemistry, self-assembly and self-organization, research on prebiotic chemistry, minimal self-replicating molecules and others. The idea of the seminar is to provide a sketch of the different ideas that form the basis of the new discipline.
The leading paradigm in chemistry so far, in particular in synthetic chemistry, has been the preparation of pure compounds. A pharmacologically active compound almost needs to be pure in order to get approval for its use as a drug. Also, it is synthetically useful to work with pure compounds as reactants as otherwise unseparably complex product mixtures form. Finally, the analytical characterization of complex mixtures is still no trivial task - despite of all progress made in separation techniques and instrumental analytics. This paradigm of the pure compound has hindered the development of complex chemical systems and the investigation of networks of chemical reactions. However, such complex mixtures can be highly interesting not only from a fundamental point of view, when the molecules involved form reactivity networks. A simple mixtures of unreactive molecules is probably not very interesting, but as soon as chemical reaction networks incorporating feedback loops and elements of non-linearity form, new, often unexpected and unpredicted so-called emergent properties arise as properties of the whole chemical system. None of the components alone has these properties, but the whole system does.
Systems chemistry is therefore an extremely interesting and very new way to do - and to think - chemistry. Beyond the fundamental insight that we can get, new materials can be developed as well as chemical systems able to adapt, react upon the action of external stimuli, and sometimes even evolve. The lecture course aims at demonstrating the intriguing emergent properties with a number of quite recent examples from the forefront of chemical research.
Another message from the course is that molecular diversity creates the New. Simple, but diverse building blocks cooperate along simple mechanisms in a system to create complex behavior. Such chemical systems can thus underline the importance of diversity and cooperation as well as the fact that complexity creates itself from simplicity without the need to involve intelligent creators, flying spaghetti monsters or anythin like this.
The Format
As learning profits from it, the course aims at maximum activity of the participants and thus does not use the traditional lecture course format. Each three-hour block is prepared and presented by a group of participants. The members of the groups take different roles. In the beginning, one member provides the course with an introduction into the topic in order to prepare the background (20 min+discussion). This is followed by the presentation of a "quickie" (15 min), which can be a brief thought experiment, a demonstration experiment, a simulation etc. Many "quickies" connect to disciplines outside chemistry to provide a glimpse of emergence in other fields. After the quicky, two particular highlight papers are discussed in detail. One member of the group takes the role of the scientist presenting his results on a conference (20 min+discussion). The task is to convince the audience of the research described in the paper. This is meant to be a detailed discussion bringing together not only the results, but also a description of the experiments that lead to the results. Another member of the group takes the role of the opponent or reviewer. His/her task is to find the weak points in the paper and to bring up doubts - just like an expert reviewer who is asked by the journal editor to evaluate a submitted manuscript. The whole group joins in this discussion of the highlight paper (20 min). For the second highlight, the roles of presenter and opponent are switched. At the end, the group summarizes the most important messages learnt (10 min).
Finally, each group has the task to prepare a short video explaining in a well-understandable and creative way their topic to a broader audience. The videos will be uploaded to a Youtube channel to make them available to a broader public. The aim of this element is to strengthen the participants ability to explain science to people outside the field. Some limited video equipment is available, but in most cases, simple equipment will probably be sufficient.
The course language will be English and thus the presentations should also be in English. Christoph Schalley offers help for the preparation of your presentations.
Schedule & Dates
The course takes place: Wednesdays, 9:15 - 12:00 am. A room or, if necessary, details on the online format will be announced shortly before the start of the course.
Grading
The quality of the presentations will be graded. This grade is averaged with the grade of the final oral exam. Appointments for the exam should be made with Christoph Schalley on a personal basis.
Seminar Topics
Please find the topics for each of the course dates below. Some keywords are provided for your orientation and some introductory literature is linked so that you have an entry into the topic. Please make sure that you do a literature search nevertheless as more recent work might have appeared meanwhile. For the literature searches, we recommend to use the Scientific Citation Index as it also allows to search for newer papers that cite a particular one that you already have. You can thus do backward as well as forward searches. Access is free from FU computers or your own computer, if you use your VPN connection.
Session 1 - Molecular Diversity in Nature - An Intro to Chemical Systems
Wed, Nov 04, 2020Christoph Schalley
Session 2 - Niklas Luhmann's Systems Theory - A Brief Introduction
Wed, Nov 11, 2020Christoph Schalley
no course on Wed, Nov 18, 2020
Session 3 - Self-Assembly on All Scales: Molecular & Meso-Scale Self-Assembly
Wed, Dec 02, 2020N.N.
Background (30 min+discussion): (Metallo-supramolecular) Self-Assembly
The principles of self-assembly illustrated by metallosupramolecular complexes such as helicates, grids, polygons, and polyeders/cages are the topic of this talk. Please discuss the general points such as error correction and reversibility as well as the three approaches to metallosupramolecular complexes including their advantages and disadvantages: Directional-Bonding Approach, Symmetry-Interaction Approach, Weak-Link Approach
Literature:
Review: B.J. Holliday, C.A. Mirkin, Angew. Chem. Int. Ed. 2001, 40, 2022
Quickie (15 min): Buttons and Wires - or when Quantity becomes Quality
Highlight 1 (30 min+discussion): Signaling Cascades in Metallo-Supramolecular Helicates
Through changes in the system - e.g. by adding new compounds as the external stimuli - the system as a whole changes, often in a predictable way, if the behavior of the components is well studied. This higlight focuses on an illustrative example for the adaptability of the systems under study.
Literature:
Highlight: V.E. Campbell et al., Nat. Chem. 2010, 2, 684
also, see: V.E. Campbell et al., Chem. Eur. J. 2009, 15, 6138
also, see: P. Mal, J.R. Nitschke, Chem. Commun. 2010, 46, 2417
Highlight 2 (30 min+discussion): Mesoscale Self-Assembly of Macroscopic Objects
The principles of chemical self-assembly can be applied to larger objects on the millimeter and centimeter scale. The talk should explain the interactions applied between the objects (capillary forces and metal alloys) and draw parallels between chemical and mesoscale self-assembly.
Literature:
Highlight: B.A. Grzybowski, H.A. Stone, G.M. Whitesides, Nature 2000, 405, 1033
also, see: G.M. Whitesides, B. Grzybowski, Science 2002, 295, 2418
also, see: M. Weck et al., J. Am. Chem. Soc. 2000, 122, 3546
also, see: S.R.J. Oliver et al., J. Am. Chem. Soc. 2001, 123, 8119
Lessons Learnt (10 min)
Session 4 - Self-Sorting
Wed, Dec 09, 2020N.N.
Background (30 min+discussion): Narcissistic vs. Social and Integrative Self-Sorting
The concepts of self-sorting: narcissitic vs. social self-sorting, the two types of social self-sorting and what distinguishes them, definition of a degree of self-sorting, completive self-sorting, integrative self-sorting
Literature:
Narcissistic/social self-sorting: P. Mukhopadhyay, A. Wu, L. Isaacs, J. Org. Chem. 2004, 69, 6157
degree of self-sorting: M. L. Saha, M. Schmittel, Org. Biomol. Chem. 2012, 10, 4651
integrative self-sorting: Z. He, W. Jiang, C. A. Schalley, Chem. Soc. Rev. 2015, 44, 779
Quickie (15 min): Granular Media and the Brazil Nut Effect
Highlight 1 (30 min+discussion): Kinetic Path Selection in Self-Sorting Systems
A central difference between self-assembly and self-sorting is that self-sorting is based on different binding motifs, each of which has its own thermodynamic and in particular kinetic characteristics. Kinetic path selection thus occurs typically during the self-sorting process, but not during self-assembly.
Literature:
Highlight: W. Jiang, P. C. Mohr, A. Schäfer, C. A. Schalley, J. Am. Chem. Soc. 2010, 132, 2309
also, see: W. Jiang, C. A. Schalley, Proc. Natl. Akad. Sci. USA 2009, 106, 10425
Highlight 2 (30 min+discussion): Self-Sorting as the Basis for Functional Molecular Assemblies
Based on metallo-supramolecular self-sorting motifs, the Schmittel group constructed molecular machinery for stimuli-responsive catalysis. Starting with the self-sorting motifs, the construction of such machines should be explained and their behavior analyzed in detail.
Literature:
Highlight: A. Goswami, T. Paululat, M. Schmittel, J. Am. Chem. Soc. 2019, 141, 15656
also, see: K. Mahata, M. L. Saha, M. Schmittel, J. Am. Chem. Soc. 2010, 132, 15933
Lessons Learnt (10 min)
Session 5 - Dynamic Combinatorial Chemistry: Self-Sorting & Mechanosensitive Libraries
Wed, Dec 16, 2020N.N.
Background (30 min+discussion): Dynamic Combinatorial Chemistry
This talk reports the approach to use reversible covalent bonds to make dynamic combinatorial libraries whose composition can change, when an appropriate template is added. How can good receptors be made with this approach? What are the limiting factors for this approach? DCLs can change their constitution, when templates are added that induce the formation of a certain library member, which binds better to the template than others. Is this a general phenomenon? What are the limitations of the dynamic combinatorial approach? Is the best binder always amplified the most or does this depend on the conditions?
Literature:
Concept: S. Otto, R.L.E. Furlan, J.K.M. Sanders, Science 2002, 297, 590
Review: R.A.R. Hunt, S. Otto, Chem. Commun. 2011, 47, 847
Quickie (15 min): The Experiment: The Transmutation of Scent
Highlight 1 (30 min+discussion): Amplification of the Wrong Receptor in Templated Dynamic Combinatorial Libraries
This highlight is intended to demonstrate that not only biomolecules such as nucleic acids or peptides can self-replicate, but also simple organic molecules do the trick.
Literature:
Highlight: Z. Grote, R. Scopelliti, K. Severin, Angew. Chem. Int. Ed. 2003, 42, 3821
also, see: K. Severin, Chem. Eur. J. 2003, 10, 2565
Highlight 2 (30 min+discussion): Mechanosensitive Dynamic Libraries
The highlight is about a very simple dynamic library, in which macrocycles form. The ring sizes depend on the mode of agitation and shaking leads reproducibly to another ring size than stirring. This mechanosensitivity should be explained in terms of the lessons learnt about systems chemistry so far.
Literature:
Highlight: J.M.A. Carnall, et al., Science 2010, 327, 1502
Lessons Learnt (10 min)
Session 6 - Self-Replication: Minimal Replicators & Logic Gates based on Networks of Replicating Peptides
Wed, Jan 06, 2021N.N.
Background (30 min+discussion): Self-Replicators: Molecular Minimal Models for Life
Small molecules with suitable structure and binding properties can act as templates for their own generation. Therefore, autocatalysis is realized so that self-replication is possible even for very simple molecules. The talk shows examples for self-replicating molecules and discusses the kinetics of self-replication. Please provide details on the peptide-based replicators as this is the basis for the second highlight.
Literature:
Fundamentals: G. von Kiedrowski, Bioorg. Chem. Frontiers 1993, 3, 113
Peptide Replicator: D.H. Lee et al., Nature 1996, 382, 525
also, see: D.H. Lee, K. Severin, Y. Yokobayashi, M.R. Ghadiri, Nature 1997, 390, 591
Quickie (15 min): Conway's Game Of Life
Highlight 1 (30 min+discussion): An Organic Minimal Replicator
This highlight is intended to demonstrate that not only biomolecules such as nucleic acids or peptides can self-replicate, but also simple organic molecules do the trick.
Literature:
Highlight: M. Kindermann, et al., Angew. Chem. Int. Ed. 2005, 44, 6750
Highlight 2 (30 min+discussion): Logic Gate Sensors Based on Self-Replication
Catalytic networks of self-replicating peptides can be used to construct chemical systems to perform Boolean logic operations. This talk describes how logic gates are constructed from such networks.
Literature:
Highlight: G. Ashkenasy et al., Isr. J. Chem. 2011, 51, 106
Lessons Learnt (10 min)
Session 7 - Biodiversity and the Stability of Ecosystems
Wed, Jan 13, 2021Prof. Britta Tietjen - Institute of Biology, Free University of Berlin
Session 8 - Nanoscale Motion: Feringa’s Molecular Motors, Janus Particles & Motion along Gradients
Wed, Jan 20,2021N.N.
Background (30 min+discussion): The Difficulties to Create Directional Motion: Feringa's Molecular Motors
The difficulties of achieving directional motion on the molecular scale are discussed. Questions are: How can molecular motors be driven? Why can motion not be obtained from heat alone? The talk describes in detail the mechanism of the light-driven motors by Ben Feringa and highlights recent applications in materials chemistry
Literature:
Review: B. L. Feringa, Angew. Chem. Int. Ed. 2017, 56, 11060
Quickie (15 min): Braitenberg Vehicles - Complexity out of Simplicity
Highlight 1 (30 min+discussion): Janus Particle-Based Molecular Motors
The talk will describe the motion of nanoparticles by gas bubble formation.
Literature:
Highlight: A. A. Solovev et al., Adv. Funct. Mater. 2010, 20, 2430
Review: S. Sanchez et al., Angew. Chem. Int. Ed. 2015, 54, 1414
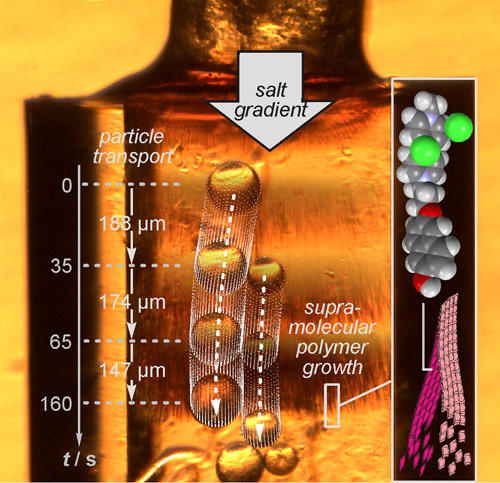
Highlight 2 (30 min+discussion): Supramolecular polymers: Transport along Gradients
Salt gradients can be used to provide a direction to the growth of supramolecular polymers. When energy is dissipated, such directional growth is capable of realizing the transport of small particles.
Literature:
Highlight: L. Cera et al., Adv. Mater. 2017, 29, 1604430
Lessons Learnt (10 min)
Session 9 - Adaptive Materials: Supramolecular Gels
Wed, Jan 27, 2021N.N.
Background (30 min+discussion): Stimuli-Responsive Supramolecular Gels
Briefly introduce what a gel and what a sol is, pointing out the differences between polymer gels and supramolecular gels and defining terms such as hydrogel, organogel, aerogel, xerogel. Then, introduce possible stimuli that may allow for external control of gel-sol transitions. Provide a few examples for such stimuli-responsive gels
Literature:
Review: C.-W. Chu, C.A. Schalley, unpublished (I will provide the literature reference in the course)
Quickie (15 min): The Funny Shampoo
Highlight 1 (30 min+discussion): Logic Gates based on Supramolecular Gels
Gels can also be made multi-stimuli responsive. This allows to implement divers logic function. As a proof-of-principle example, a supramolecular gelator is presented here which - depending on the other ingredients - allows us to construct seven different two- and even three-input logic gates.
Literature:
Highlight 2 (30 min+discussion): Energy-Gradient Driven Transient Assembly of Supramolecular Gels
If a supramolecular gelator is incorporated in a reaction network, a transient, non-linear and non-equilibrium formation of supramolecular gels can be achieved.
Literature:
Highlight: J. Boekhoven, W.E. Hendriksen, G.J.M. Koper, R. Eelkema, J.H. van Esch, Science, 2015, 349, 1075
Lessons Learnt (10 min)
Session 10 - Oscillating Reactions
Wed, Feb 03, 2021Christoph A. Schalley
Background (30 min+discussion): The Belousov-Zhabotinski Reaction Network
Autocatalysis and feedback loops are an important topic in the Belusov-Zhabotinski reaction. As long as chemical energy generated through the individual steps of this reaction flows through the system, patterns are created. When all reactants are consumed and the energy flow stops, the patterns are destroyed through diffusion. This reaction is a great chemical example for spontaneous pattern generation in open systems.
Literature:
Review: I.R. Epstein, K. Showalter, J. Phys. Chem. 1996, 100, 13132
Review: R.J. Field, F.W. Schneider, Chem. unserer Zeit 1988, 22, 17 (German)
Quickie (15 min): The BZR Experiment
Highlight 1 (30 min+discussion): Oscillating Reactions to Drive Transport
The oscillations in the BZ reaction are driven by a flow of energy: Chemical energy is dissipated into heat in this process. This gradient can be used to drive unidirectional processes such as the transport of particles on the surface of gels, peristaltic motion in a gel tube or an autonomously walking gel. Before you describe the transport phenomena, make sure that everyone has understood, what a gel is and what "swelling" is. Then explain the transport phenomenon on a molecular level.
Literature:
Highlight: S. Maeda, et al., Adv. Mater. 2007, 19, 3480
Review: R. Yoshida, Adv. Mater. 2010, 22, 3463
Highlight 2 (30 min+discussion): Quorum Sensing in Chemical Systems
Oscillating reactions can be performed on particles, when the redox catalyst is fixed to these. Each particle has its own "blinking" frequence. However, when a critical group size in an ensemble of particles is reached, the blinking occurs in a cooperative fashion.
Literature:
Highlight: A.F. Taylor et al., Science, 2009, 323, 614
also, see: M.R. Tinsley et al., Phys. Rev. Lett. 2009, 102, 158301
Lessons Learnt (10 min)
Session 11 - Origin of Life: Homochirogenesis & Synthesizing Life
Wed, Feb 10, 2021N.N.
Background (30 min+discussion): The Origin of Homochirality
It would be good to present the different theories about the origin of homochirality in the background talk. There are biotic and abiotic theories that involve statistical fluctuations with autocatalytic amplification (non-deterministic theories), chirality through non-symmetric processes on elementary particle level (deterministic theories)? There are quite many potential explanations for the development of homochirality. The talk should first provide a breakdown of the three different steps involved (1. symmetry breaking, 2. chiral amplification of one particular type of molecule and 3. chirality transfer to other types of molecules) and then discuss the different theories for symmetry breaking.
Literature:
Review: W.A. Bonner, Origin Life Evol. Biosphere 1992, 21, 407
Parity violation: R.A. Hegstrom, D.K. Kondepudi, Spektrum der Wissenschaft 1990, 56
Statistical fluctuations: M. Avalos et al., Chem. Commun. 2000, 887
Quickie (15 min): AntSim: The Organization of Ant Nests
Highlight 1 (30 min+discussion): Chiral Self-Replication and Asymmetric Autocatalysis#
Minimal model self-replicators can amplify chirality when a chiral template is formed from achiral precursors. The talk describes in detail examples for this finding.
Literature:
Highlight: V.C. Allen, D. Philp, N. Spencer, Org. Lett. 2001, 3, 727
also, see the Soai reaction for an example of chiral autocatalysis: K. Soai, T. Shibata, I. Sato, Acc. Chem. Res. 2000, 33, 382
Highlight 2 (30 min+discussion): Synthesizing Life
If one accepts vesicles as minimal models for cells, coupling simple reactions to the formation and degradation of the membrane-forming components with the growth and division of the vesicles. It would be good to first introduce the terms homeostasis and autopoiesis - (Francisco Varela and Humberto Maturana). In view of the following talk, please restrict this one to laying the foundation and providing few and simple examples. Discuss the attempts to synthesize simple protocells that can evolve. How far have chemists already gone? What is left to do?
Literature:
Highlight: H.H. Zepik, E. Blöchliger, P.L. Luisi, Angew. Chem. Int. Ed. 2001, 40, 199#
Review: J.W. Szostak, D.P. Bartel, P.L. Luisi, Nature, 2001, 409, 387
Lessons Learnt (15 min)
Session 12 - Video Presentations, Summary & Conclusions
Wed, Feb 17, 2021All Participants