Template Effects for Efficient Rotaxane Synthesis
The stastistical synthesis of rotaxanes is extremely inefficient. Therefore, non-covalent template effects are used to preorganize the rotaxane axle and its wheel in a threaded pseudorotaxane-like fashion. In the following step, bulky stoppers are attached to the axle in order to prevent it from deslipping. A mechanical bond is thus created.
We mostly use the crown ether/secondary ammonium ion motif and hydrogen bonded complexes of tetralactam macrocycles and amide or diamide stations. The group developed two new template effects, one based on an axle incorporating a phenolate anion, one utilizing a diketopiperazine axle center piece (see Figure). The diketopiperazine is held inside the wheels cavity by four hydrogen bonds.
Some of the rotaxanes are switchable. The phenolate axle can be protonated and deprotonated and then acts as a "molecular brake". When stopper attachment to the deketopiperazine axle is carried out by click chemistry, the wheel is located at the diketopiperazine station, but the addition of chloride shifts it to one of the two triazole stoppers.
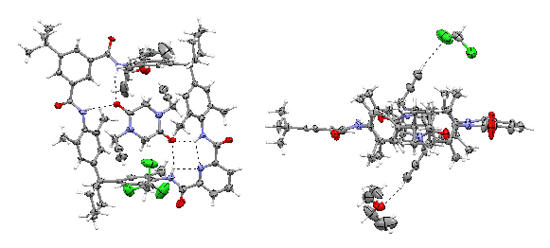
Top and side view of a solid-state structure of a pseudorotaxane with a diketopiperazine axle threading through a tetralactam macrocycle
Related Publications
- 158
CH•••O Hydrogen Bonds in "Clicked" Diketopiperazine-Based Amide Rotaxanes
E.V. Dzyuba, L. Kaufmann, N.L. Löw, A.K. Meyer, H.D.F. Winkler, K. Rissanen, C.A. Schalley
Org. Lett. 2011, 13, 4838-4841
- 126
Conformational Flexibility of Tetralactam Macrocycles and their Intermolecular Hydrogen-Bonding Patterns in the Solid State
S.S. Zhu, M. Nieger, J. Daniels, T. Felder, I. Kossev, T. Schmidt, M. Sokolowski, F. Vögtle, C.A. Schalley
Chem. Eur. J. 2009, 15, 5040-5046
- 119
Templated Synthesis of Interlocked Molecules
C. A. Schalley, J. Illigen in: Bottom-up Nanofabrication: Supramolecules, Self-Assemblies, and Organized Films, K. Ariga, H.S. Nalwa (Eds.), American Scientific Publishers, Valencia/USA, 2009
- 117
A Modular "Toolbox" Approach to Flexible Branched Multi-Macrocyclic Hosts as Precursors for Multiply Interlocked Architectures
B. Baytekin, S.S. Zhu, B. Brusilowskij, J. Illigen, J. Ranta, J. Huuskonen, K. Rissanen, L. Kaufmann, C.A. Schalley
Chem. Eur. J. 2008, 14, 10012-10028
- 90
Helicate, Macrocycle, or Catenate: Dynamic Topological Control over Subcomponent Self-Assembly
M. Hutin, C. A. Schalley, G. Bernardinelli, J. R. Nitschke
Chem. Eur. J. 2006, 12, 4069-4076
- 82
Controlling the rate of shuttling motions in [2]rotaxanes by electrostatic interactions: a cation as solvent-tunable brake
P. Ghosh, G. Federwisch, M. Kogej, C. A. Schalley, D. Haase, W. Saak, A. Lützen, R. Gschwind
Org. Biomol. Chem. 2005, 2691-2700
- 46
Novel template effect for the preparation of [2]rotaxanes with functionalised centre pieces
P. Ghosh, O. Mermagen, C. A. Schalley
Chem. Commun. 2002, 2628-2629


