Ultraviolet Photodissociation Initiated Radical Chemistry Enables Facile Glycan Isomer Identification
Riggs, D.; Hofmann, J.; Seeberger, P. H.; Pagel, K.; Julian, R.* – 2018
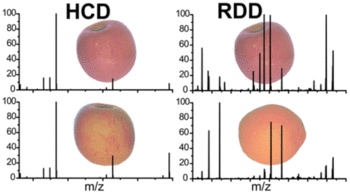
Glycans are fundamental biological macromolecules, yet despite their prevalence and recognized importance, a number of unique challenges hinder routine characterization. The multiplicity of OH groups in glycan monomers easily afford branched structures and alternate linkage sites, which can result in isomeric structures that differ by minute details. Herein, radical chemistry is employed in conjunction with mass spectrometry to enable rapid, accurate, and high throughput identification of a challenging series of closely related glycan isomers. The results are compared with analysis by collision-induced dissociation, higher-energy collisional dissociation, and ultraviolet photodissociation (UVPD) at 213 nm. In general, collision-based activation struggles to produce characteristic fragmentation patterns, while UVPD and radical-directed dissociation (RDD) can distinguish all isomers. In the case of RDD, structural differentiation derives from radical mobility and subsequent fragmentation. For glycans, the energetic landscape for radical migration is flat, increasing the importance of the three-dimensional structure. RDD is therefore a powerful and straightforward method for characterizing glycan isomers.