Peptide Aggregation
Peptides and proteins are able to adopt more than one thermodynamically stable conformation, a property associated with many neurodegenerative conditions, such as Alzheimer’s, Huntington’s, and Creutzfeldt-Jakob disease. In these cases, the changes in protein secondary and tertiary structure are the cause of so-called plaque formation. When deposited in nerve tissues, these insoluble protein aggregates can cause often devastating symptoms of dementia. In spite of having been the focus of numerous scientific studies, the formation of fibrils from monomeric and oligomeric peptide building blocks is not yet understood. By means of the above-described peptide model systems, our lab has studied the role of electrostatic interactions, metal ions, and post-translational modifications in aggregation processes at the molecular level (Pagel, Curr. Opin., 2008; Falenski, Chem. Eur. J., 2010). Our current projects in this area are described below.
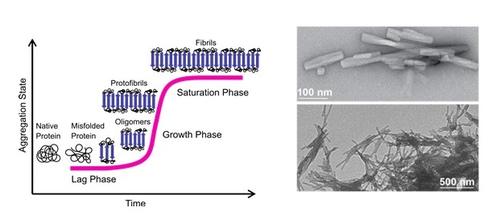
(Left) Pathway of amyloid formation (nucleation-dependent fibril formation). (Right) Negative staining transmission electron microscopy (TEM) images of an NFGAIL solution showing the morphology of the resulting amyloid fibrils.
Image Credit: Koksch Group
Under conditions of environmental stress native proteins can undergo misfolding and aggregation. A well-known example of highly ordered misfolded protein aggregates is amyloid fibrils. These nearly insoluble and fibrillar composites are defined as self-assembled supermolecules with a characteristic cross-β structure. Moreover, extracellular amyloid plaques are pathological signatures of neurodegenerative disorders like Alzheimer's disease (AD), Huntington’s disease (HD), Parkinson's disease (PD) and type II diabetes (T2D). The formation of amyloid fibrils is considered as a multi-step reaction characterized by a variety of transient oligomers and polymorphic fibril structures. Investigation of individual reaction steps and aggregate species is significant for understanding the nucleation of amyloid fibrils and the driving forces involved in their formation, as well as to develop techniques to regulate their kinetics.
Our work focuses on the highly amyloidogenic fragment NFGAIL derived from the human islet amyloid polypeptide (hlAPP). To gain insights into the early oligomer properties we studied NFGAIL by using ion-mobility mass spectrometry (IM-MS) coupled with infrared multi-photon dissociation spectroscopy (IRMPD), as well as solution techniques. We found that at neutral pH NFGAIL follows a nucleation-dependent mechanism to form amyloid fibrils. During the lag phase, highly polydisperse, polymorphic and compact oligomers, as well as extended intermediates, are present. We gathered experimental data for individual amyloid oligomeric states and conformations during the onset of fibril formation for NFGAIL oligomers.
In further work, we aim to incorporate halogenated Phe residues into NFGAIL via solid phase peptide synthesis. We will use thioflavin T-(ThT) fluorescence staining, transmission electron microscopy (TEM) and solid-state 19F-NMR spectroscopy to study the impact of subtle changes in electron density on the amyloid folding kinetics. Our collaborators include Harmut Oschkinat (FMP), Kevin Pagel (FU Berlin), Christoph Schütte (FU Berlin), Ralf Kornhuber (FU Berlin), and Andreas Thünemann (BAM).
Further Reading
[1] W. Hoffmann, K. Folmert, J. Moschner, X. Huang, H. von Berlepsch, B. Koksch, M. T. Bowers, G. von Helden, K. Pagel, Journal of the American Chemical Society 2018, 140, 244-249. DOI: 10.1021/jacs.7b09510
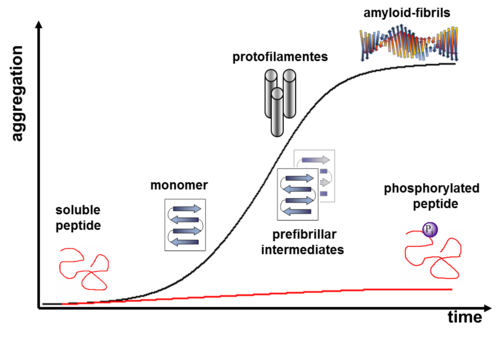
Phosphorylation is one of the most important postranslational protein modifications in nature. Investigating the impact of the electronegative phosphate group on the conformation of peptides is of great interest for the elucidation of many pathologic events, such as tauopathies. Depending upon the location of this covalent modification, and the neighboring functional groups in the peptide, enzymatic phosphorylation can either stabilize or destabilize specific conformational states (Broncel, Chem. Eur. J., 2010).
We have shown that incorporation of phosphoserines into a 26-residue, amyloid-forming model peptide precludes amyloid formation, and that enzymatic dephosphorylation of these peptides with phosphatase lambda (λ-APPase) can induce a conformational switch from a coiled coil structure to amyloids (Broncel, Chem. Eur. J., 2010; Broncel, Chem. Commun. 2010). Furthermore, we developed a peptide system that enables detailed amyloid-aggregation inhibition studies using cAMP dependent Proteinkinase A (PKA) (Figure 2). Further systematic studies of this kind will contribute significantly to our understanding of how phosphorylation influences pathways leading to peptide aggregation, and which structural interactions affect the conformation of a peptide during this process.