Regulation of cartilage differentiation
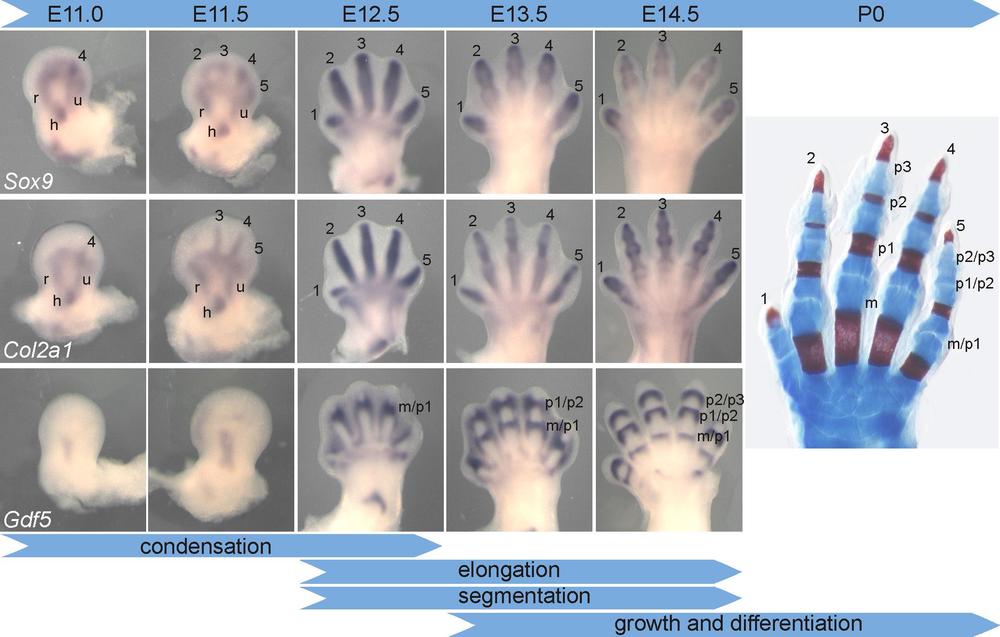
Our hand skeleton develops in a series of interconnected developmental steps that lead from the initial emergence of cartilaginous condensations in the autopode to their expansion and elongation and their segmentation by joints. Concomitantly, the phalanges are separated by regression of the interdigital tissue. These events are orchestrated by a complex regulatory network that assures the correct positioning and growth of the skeletal elements. Disturbances in this network, caused for example by gene mutation, results in hand malformation syndromes.
The figure shows the formation, elongation and segmentation of the digits in the mouse forelimb. Left panel: whole-mount in-situ hybridization with probes showing precartilaginous condensations (Sox9), cartilage condensations (Col2a1) and joint interzones (Gdf5). Right panel: skeletal preparation of a newborn mouse hand. Digits 1-5 are indicated, m=metacarpal, p1-p3=phalanges 1-3. From Stricker and Mundlos, Developmental Dynamics 2011, 240(5), 990-1004.
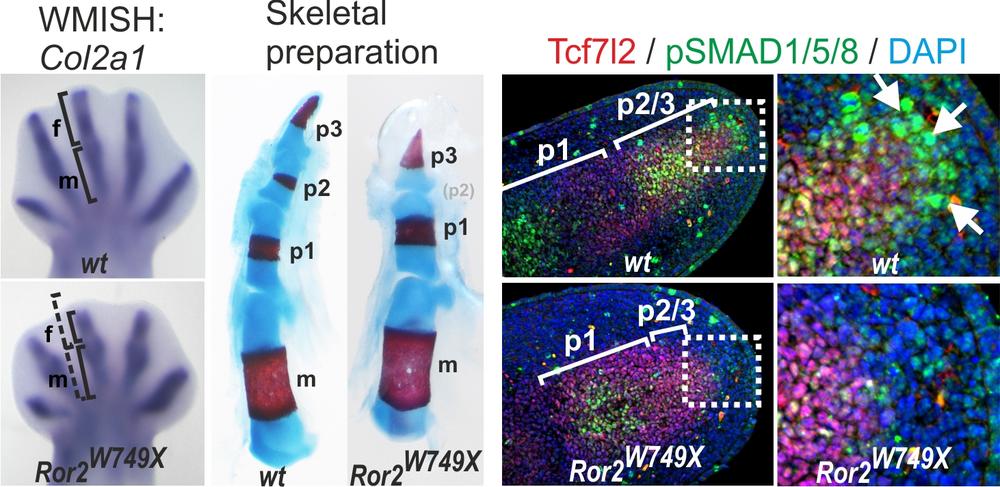
To elucidate the genetic mechanisms of digit formation and elongation we analyzed a group of human hand malformations, the brachydactylies (Greek, brachys=short, dactylos=finger) as well as other inheritable conditions. In our analysis we focus on the most severe form of the brachydactylies, brachydactyly type B1. BDB1 is characterized by shortening/hypoplasia/aplasia of the distal phalanges, is caused by mutations in ROR2, encoding a receptor tyrosine kinase. Analyzing a knock-in mouse mutant harboring an exact copy of a human BDB mutation (Ror2-W745X, Raz et al. 2008), we identified a specific impairment of digit elongation during the formation of the phalangeal condensations. This was caused by a disruption of Bone morphogenetic protein (BMP) signaling in the so-called “phalanx-forming region”, a signaling center previously identified in the chicken. Our results provide genetic evidence that this signaling center is active in mammalian digit elongation and suggest that its failure is causative for human disease (Witte et al. 2010).
Phenotype of the Ror2-W749X knock-in mouse mutant. Col2a1 WMISH shows a defect in the elongation of the digit anlagen at E13.5. Skeletal preparations of forelimbs from newborn mice, cartilage stains blue, bone stains red. Note the missing medial phalanges in the W749X mutant (arrows). Immunostaining for pSMAD1/5/8 demonstrates the presence of a BMP signaling center distal to the growing cartilage condensation, which is absent in the Ror2-W745X mouse. Modified from Witte et al. 2010
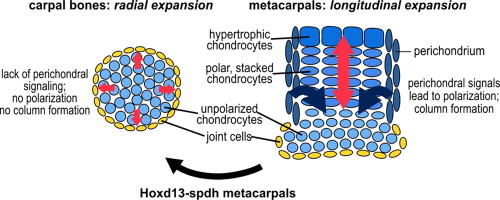
Our appendicular skeleton mainly consists of long bones with exception of the wrist. Directional expansion of long bones is achieved via the so-called growth plate, a structure in which chondrocytes undergo a series of stereotypical differentiation events until they are ultimately replaced by bone. Importantly, in this growth plate chondrocytes in parallel undergo a cellular movement that leads to lateral restriction and concomitant elongation of the anlage in a process similar to convergent extension movements seen in gastrulation. It was noted, that in mice with mutations in the transcription factor HOXD13, which are a model for human Synpolydactyly (SPD), metacarpals ate homeotically transformed to carpal bones (Villavicencio-Lorini et al. J. Clin. Invest. 2010). We have analyzed the molecular mechanism behind this transformation and found that HOXD13 is required for proper spatial induction of the non-canonical WNT ligand WNT5A. This signaling in turn is required for polarization of growth plate and perichondral cells and together confers longitudinal growth to skeletal elements (Kuss et al. Dev. Biol 2014).
Perichondral signaling is required for bidirectional versus radial growth of cartilage elements; from Kuss et al. 2014.
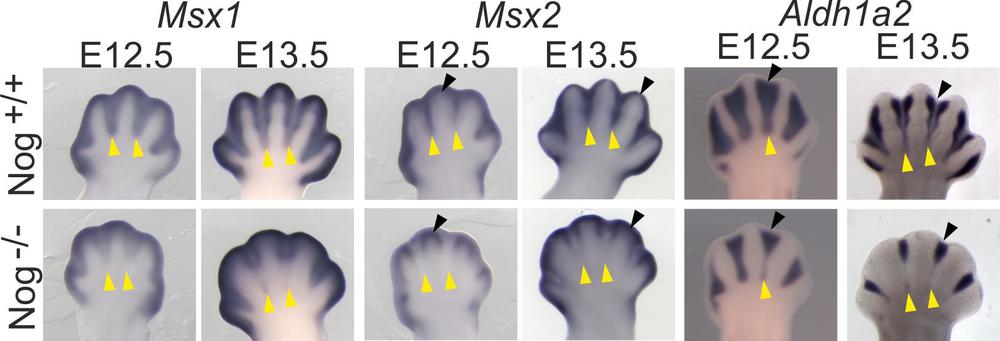
Individual digits separate during development via regression of the interdigital tissue, mostly by apoptosis. It is generally thought that interdigital apoptosis in controlled by the BMP signaling pathway. Surprisingly, we found that mice mutant for the BMP antagonist Noggin showed a phenotype of impaired interdigital regression.
Failure of interdigit marker expression in Noggin mutants analyzed by whole-mount in-situ RNA hybridization. From Murgai et al. 2018.
Noggin deficient embryos showed a remarkably increased expression of the signaling molecule Indian hedgehog (Ihh). Embryos deficient for Ihh showed a converse phenotype, i.e. increased apoptosis in interdigital tissue. This indicates that exacerbated Ihh signaling could prevent interdigit regression, while in normal development a low dose of Ihh singlaing in the interdigits is required to prevent aberrant apoptosis (Murgai et al. 2018).