Scaffolding proteins at the membrane
Dynamic Palmitoylation in CD4+ T Cells
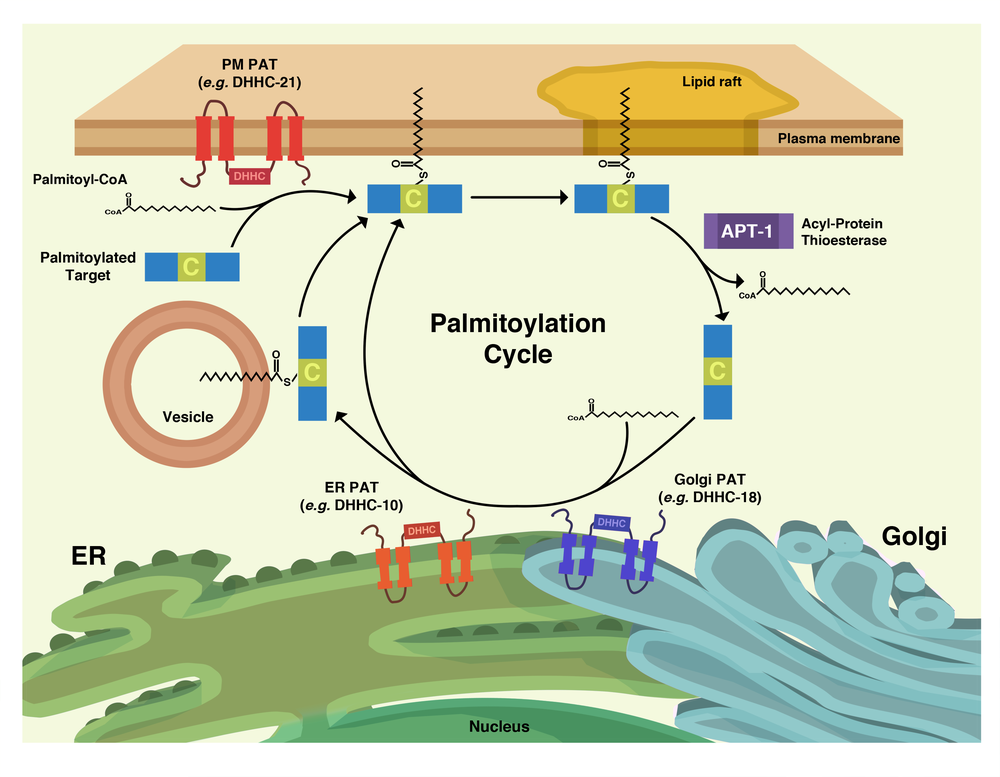
Palmitoylation is the posttranslational addition of 16-carbon fatty acid chains to reduced thiol groups of cysteine residues. While thioester exchange can lead to spontaneous palmitoylation, since the early 2000’s it has come to light that this modification is catalyzed by the DHHC family of palmitoyl acyltransferases (PATs). However, unlike irreversible lipid modifications such as myristoylation or prenylation, the relatively labile thioester bond of palmitoyl groups allows for enzymatic removal by a class of enzymes known as palmitoyl thioesterases.
This reversible nature allows for the possibility that palmitoylation may have arisen as a general cellular regulatory mechanism, allowing proteins to be localized to and from liquid-ordered membrane microdomains (e.g. “lipid rafts”) via enzymatic addition or removal of palmitoyl groups, respectively. Indeed, a number of palmitoylated proteins, especially in a neuronal context, have been established as participating in so-called “palmitoylation cycles”; perhaps the most well known examples are the Galpha G protein subunits and small GTPases, including N-Ras and H-Ras.
Our work focuses on palmitoylation’s role as a “molecular switch” in a CD4+T cell context. As phosphorylation is a well established post-translational molecular switch used by T cells in a number of important cellular events on a seconds timescale, we seek to draw a parallel with palmitoylation on a minutes timescale.
Collaboration Partners:
- AG Krause, FMP
- AG Kliche, Otto-von-Guericke-University Magdeburg
- AG Brügger, Universität Heidelberg
Regulation of SH3 domain-containing scaffolds in endocytosis and synaptic vesicle cycling
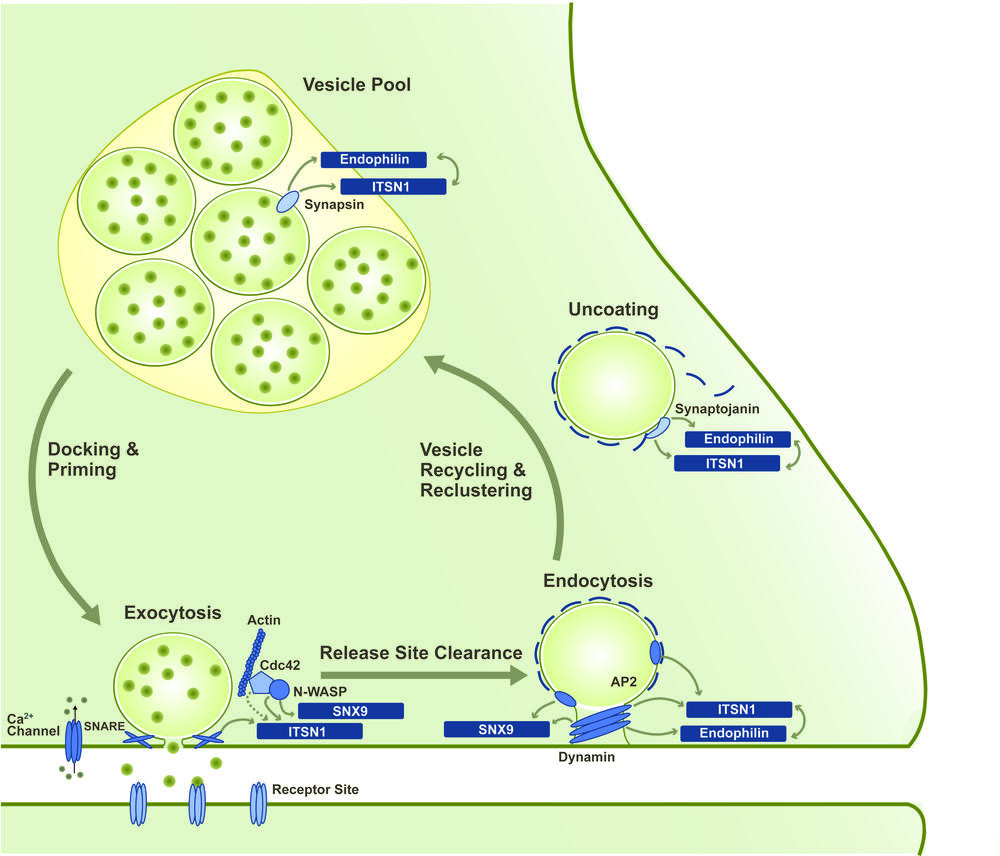
Fast signaling between neurons is based on the stimulation-evoked release of neurotransmitter molecules into the synaptic cleft. Interaction of these molecules with the receptors of the postsynapse then triggers a new action potential in the neighboring neuron. The release of the neurotransmitter via the exocytosis of synaptic vesicles (SVs) is followed by compensatory endocytic retrieval of the excess vesicular membrane. Newly formed SVs are then clustered in specialized vesicle pools from where they are recruited to the membrane for a new round of release. This repeated sequence of events is referred to as the synaptic vesicle cycle.
In order to sustain synaptic transmission even during phases of high neuronal activity, the individual processes of the synaptic vesicle cycle need to be tightly coupled and regulated in an activity-dependent manner. However, the molecular principles underlying this coupling still remain elusive. Interesting candidates for central regulatory roles in the synaptic vesicle cycle are SH3-domain-containing adaptor and scaffolding proteins such as intersectin 1 (ITSN1), endophilin A1 and SNX9. These primarily endocytic proteins shuttle between the sites of SV exocytosis, endocytosis and clustering and interact with a variety of proteins from all stages of the vesicle cycle via their SH3 domains [3].
Our work focuses on the mechanistic and regulative principles that allow the versatile role of these SH3-domain-containing proteins within the synaptic vesicle cycle. We apply structural biology and biophysical methods to analyze the inter- and intramolecular interactions mediated via the SH3 domains. In close cooperation with the AG Haucke at the Leibnitz Institute for molecular pharmacology (FMP), the functional consequences of these interactions are also studied in a biologic context, e.g. by applying mouse models. Fabian Gerth currently works on this project that is part of the SFB958(A07).
Collaboration Partners:
- AG Haucke, FMP